Check
Certificates and go to Uniqa Shop
Certificates and go to Uniqa Shop

Our Certificates
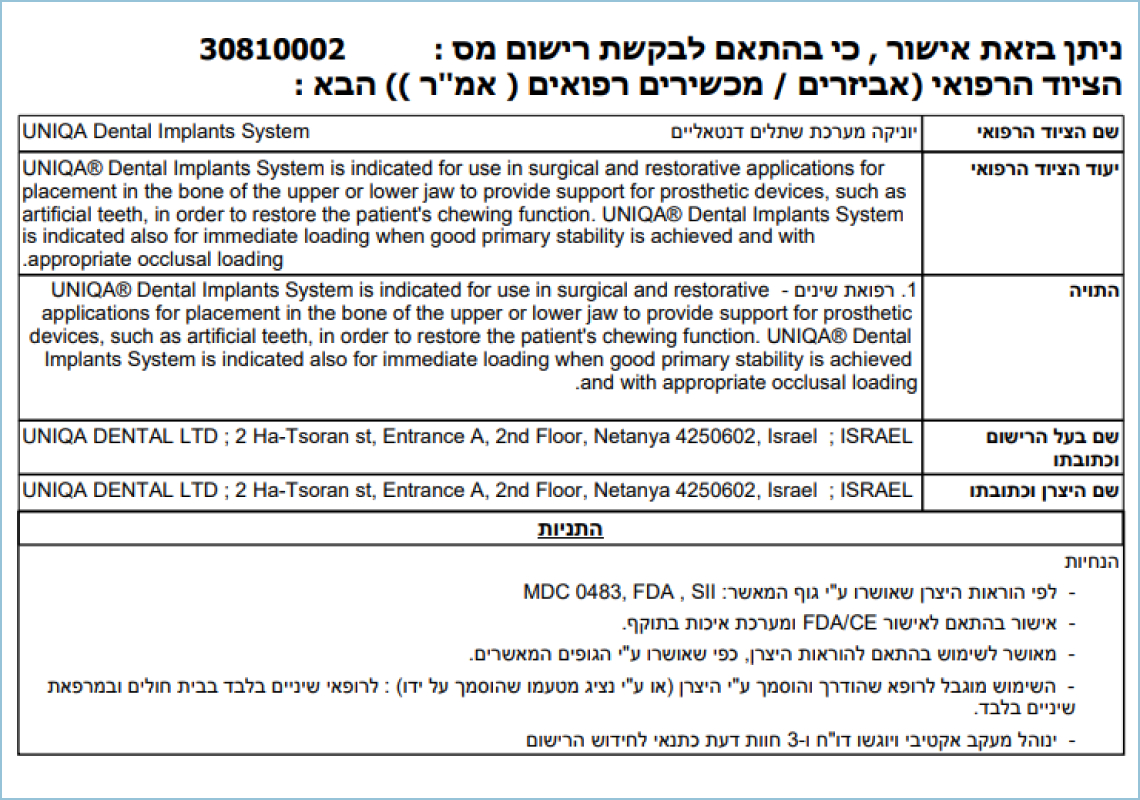
Uniqa Dental AMAR
This document serves as confirmation of the registration and tax compliance of Uniqa Dental for medical components for dental implantation. The Uniqa Dental AMAR Certificate is issued by the Ministry of Health of Israel within the Department of Medical Equipment.The document allows the use of components for dental implantation according to the manufacturer's instructions. The permission is valid until October 21, 2024.

Uniqa Dental FDA K180159
This document signifies the official approval granted by the Food and Drug Administration (FDA) for the sale and distribution of dental implants dedicated to the restoration of teeth in the United States. The certification is granted to Uniqa Dental, acknowledging their compliance with the regulatory requirements and adherence to quality and safety standards.
Open in new window
Our Certificates of Analysis
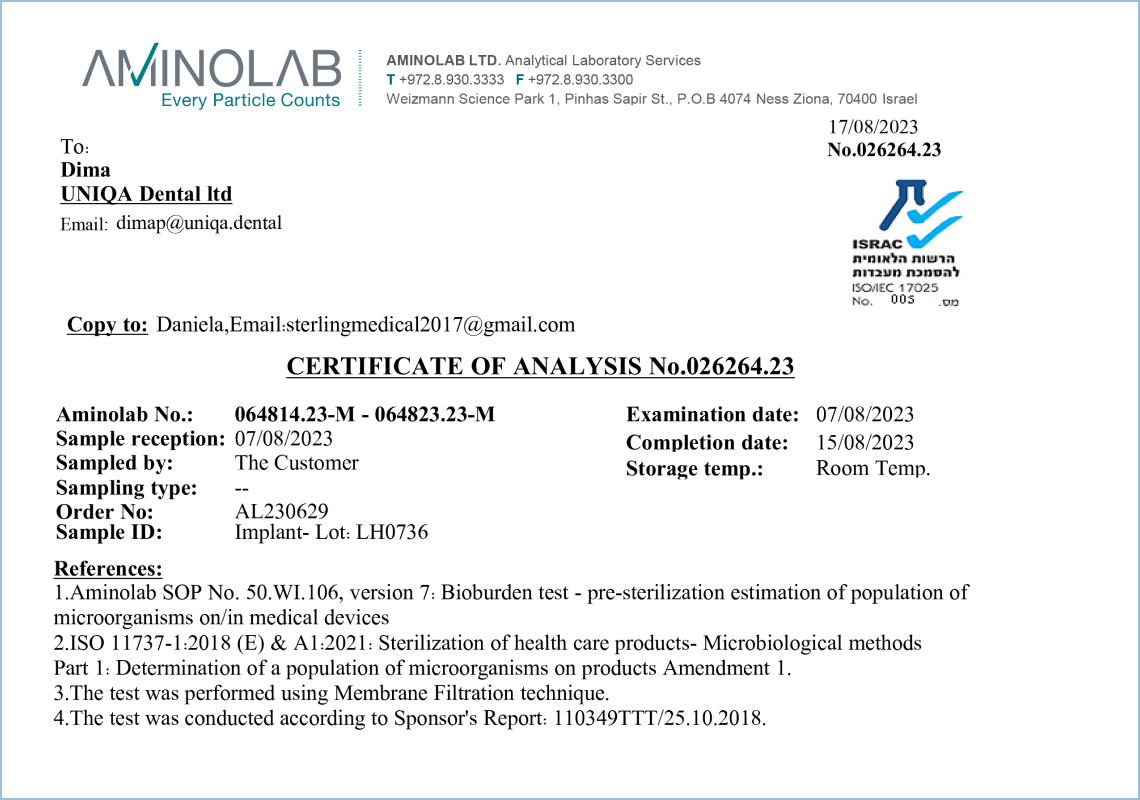
Bioburden
This certificate confirms that ten product samples have undergone laboratory testing at the certified laboratory AMINOLAB LTD (Israel). The laboratory is accredited under ISO/IEC 17025 with standard operating procedures. The research results confirm that Uniqa Dental products can be used in the medical field for direct purposes without restrictions.
Open in new window

LAL
This certificate confirms that Uniqa Dental implants have undergone laboratory testing at the certified laboratory AMINOLAB LTD (Israel). The laboratory is accredited under ISO/IEC 17025 with standard operating procedures. The research results confirm that toxin levels do not exceed threshold values, and the products can be used for medical purposes, including direct contact with the sterile environment of the body.
Open in new window
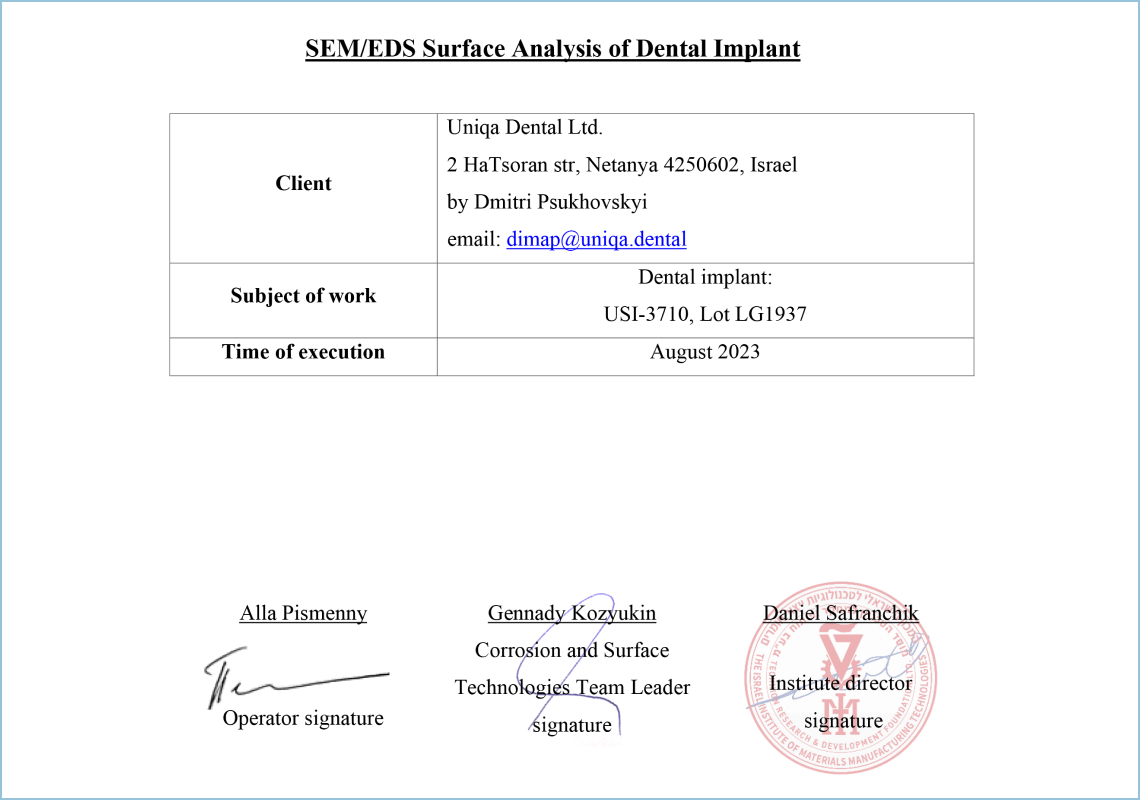
SEM
This protocol contains the results of the implant surface analysis using scanning electron microscopy (SEM) supplemented with energy-dispersive X-ray spectroscopy (EDS). The implant sample was provided by the Israel Institute of Materials Production Technologies (IMT). The study analyzed the surface structure of the implant and verified the presence and chemical composition of impurities on the implant surface.
Open in new window

XPS
This protocol contains the results of the investigation of the chemical composition and electronic state of the implant surfaces. The XPS/AEA analysis methodology was used, where XPS stands for X-ray photoelectron spectroscopy, and AEA stands for Auger electron spectroscopy. Both methods are based on measuring the energy of electrons emitted from the sample under the influence of X-ray or electron beams. The samples for the study were provided at the Ilse Katz Institute for Nanoscale Science and Technology.
Open in new window
Quality:
UNIQA Dental has established, implemented, and communicated throughout the organization a Medical Devices Quality Management System that meets customer requirements, Regulatory & International Standards Requirements - ISO 13485:2016, EU MDR 2017/745, USA QSR 21 CFR 820. Since its establishment, UNIQA Dental's philosophy has been impeccable quality, which speaks for itself. The emphasis is on quality. We focus on equipment precision and the quality of materials from which dental implants and other dental restoration elements are made. This has enabled UNIQA Dental to achieve great results in the manufacture of dental implants, multi-units, abutments, and other prosthetic parts with minimum tolerances (deviation of the actual size). This is a key quality criterion. The accuracy of fit and gap-free fit, especially at the implant/abutment line, is important.
Medical regulation:
UNIQA Dental's quality management system meets customer requirements as well as regulatory & international standards. Requirements: - ISO 13485:2016, EU MDR 2017/745, USA QSR 21 CFR 820. Our dental Implant system has been cleared for marketing in the USA under 510k K180598. UNIQA Dental LTD's medical devices that are sold in the EU bear the CE Mark.
Disclaimer:
The libraries provided are valid for EU market customers. Please note that certain products referenced on this website may not be cleared for regulatory approval, distribution, or sale in your market. Availability, regulatory status, and usage of products vary by region. It is the responsibility of the customer to ensure compliance with all applicable local and international regulations. For specific inquiries, please contact your local sales representative or distributor.
Total manufactured
IMPLANTS
0
MULTI-UNIT ABUTMENTS
0
ANGLED MULTI UNIT ABUTMENT
0
Our products compatible with















-
Screw Retained Restoration + Dental Implant Digital CAD/CAM Trial Kit
Original price was: $200.$9.90Current price is: $9.90. Buy Now -
Screw Retained Restoration Digital CAD/CAM Trial Kit
Original price was: $122.$0.99Current price is: $0.99. Buy Now

-
Hot
-
Hot
-
Hot
-
Hot
-
Hot










