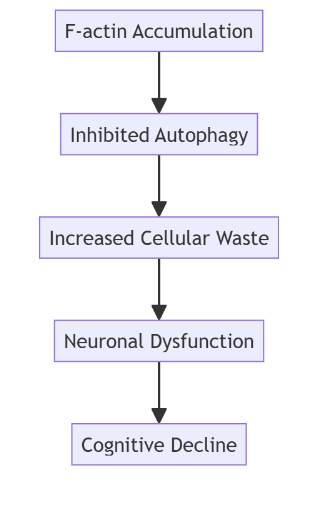
Brain aging and the fight against it: scientists have managed to stop the accumulation of F-actin protein in fruit flies (prospective study)
We’ve written before about the link between oral health and the risk of developing Alzheimer’s disease. And here’s new research where they’ve found a way to slow the accumulation of filamentous actin (F-actin) in the brain, which causes cognitive decline as we age. And this study is interesting in itself, because each of us wants to stay active in old age. The results, published in Nature Communications, have provided a deeper understanding of how this common structural protein affects neuronal health and autophagy, opening a promising avenue for developing new interventions that promote healthier aging. This article explores the complex relationships between F-actin accumulation, impaired cellular recycling, and the potential impact on human cognitive health.
What Is F-actin and Why Does It Matter?
F-actin is a polymerized form of actin, a protein that plays a vital role in maintaining the shape and function of cells. Actin filaments are crucial for various cellular activities, including movement, division, and intracellular transport. However, as the brain ages, the accumulation of F-actin disrupts cellular processes, particularly autophagy—a critical mechanism for clearing out damaged and unnecessary components.
Key Role of F-actin in Cellular Structure
Actin proteins form a complex network of filaments that support the cellular architecture. In neurons, F-actin is involved in synaptic function and plasticity, which are essential for learning and memory. The excess buildup of F-actin, however, can interfere with these processes, leading to a decline in cognitive performance.
How F-actin Buildup Impairs Autophagy in Aging Brains
Autophagy, often referred to as the “cellular garbage disposal system,” is the body’s way of clearing out damaged proteins, lipids, and organelles. This process is vital for maintaining cellular health and function. With aging, the efficiency of autophagy diminishes, but the exact reasons have remained unclear—until now. The new study identifies F-actin buildup as a key factor inhibiting autophagy, particularly in the neurons of aging brains.
Mechanism of F-actin Interference
The researchers found that when F-actin accumulates in the brain, it obstructs autophagic pathways, preventing the breakdown and recycling of cellular waste. This interference results in the accumulation of harmful debris, including damaged DNA, proteins, and cellular organelles, which impairs neuronal function and accelerates cognitive decline.
Diagram: Impact of F-actin Accumulation on Autophagy
Genetic Interventions to Prevent F-actin Buildup
Leveraging the well-mapped genome of fruit flies, the researchers used targeted genetic interventions to reduce F-actin accumulation. By modifying specific genes such as Fhos, which is responsible for elongating and organizing actin filaments, they were able to prevent the buildup of F-actin in aging neurons. The intervention led to a significant improvement in the flies’ cognitive abilities and extended their healthy lifespan by 25-30%.
Key Genetic Findings
- Gene Targeting: The researchers identified Fhos as a critical gene influencing F-actin buildup. By reducing its expression, they prevented the accumulation of F-actin in the brain.
- Improved Autophagy: Genetic modification not only reduced F-actin levels but also reactivated autophagic pathways, restoring the brain’s ability to clear out cellular waste.
- Enhanced Lifespan and Healthspan: The fruit flies showed improved learning and memory, increased activity levels, and better overall health, indicating that preventing F-actin buildup has a broad positive impact on aging.
Diet and Pharmacological Interventions: Rapamycin and Dietary Restriction
The study also explored non-genetic interventions to reduce F-actin accumulation. Two notable approaches were dietary restriction and the use of rapamycin, a drug known to extend lifespan.
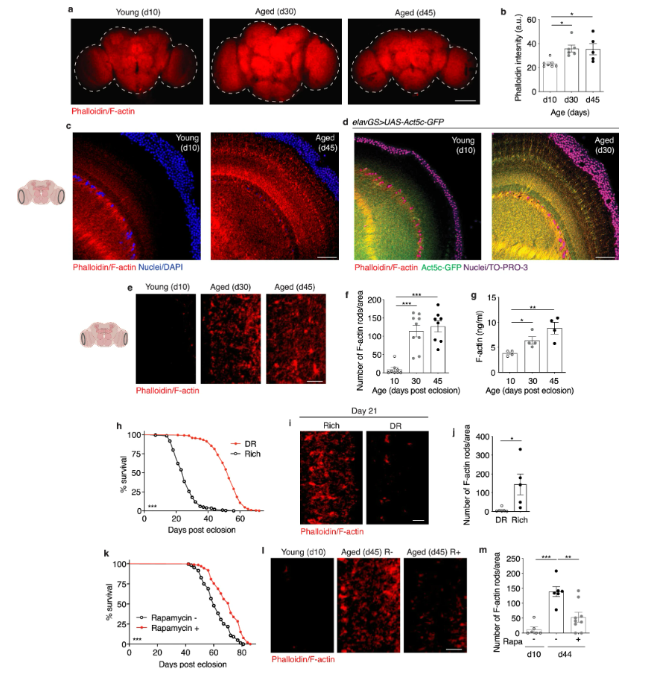
A immunostaining of brains at 10x magnification from young (10-day-old) and aged (30-day-old and 45-day-old, as indicated) canton s flies, showing f-actin fluorescence intensity (red channel, phalloidin). Scale bar is 100 µm. B quantification of mean phalloidin fluorescence intensity in brains as shown in a. D10 n = 7, d30 n = 6, and d45 n = 5 flies, as indicated. *p (d10 vs. D30) = 0. 0158, *p (d10 vs. D45) = 0. 0263; one-way anova, tukey’s multiple comparisons test. C immunostaining of brains at 63x magnification from young (10-day-old) and aged (45-day-old) flies, showing f-actin (red channel, phalloidin) and nuclear dna (blue channel, dapi). These findings were repeated in more than 15 independent experiments. Scale bar is 20 µm. Accompanying diagram indicates brain region where imaging was conducted. D immunostaining of brains from young (10-day-old) and aged (30-day-old) elavgs > uas-act5c-gfp flies, showing f-actin (red channel, phalloidin), act5c-gfp (green channel), and nuclear dna (blue channel, to-pro-3). These findings were repeated in 3 independent experiments. Scale bar is 20 µm. E immunostaining of brains at 63x magnification from young (10-day-old) and aged (30-day-old and 45-day-old, as indicated) canton s flies, showing f-actin-rich rods (red channel, phalloidin). Scale bar is 5 µm. Accompanying diagram indicates brain region where imaging was conducted. F quantification of f-actin-rich rods per 1 mm2 area of brain optic lobes as shown in e. D10 n = 8, d30 n = 9, d45 n = 8 flies, as indicated. ***p (d10 vs d30 and d10 vs d45) < 0. 0001; one-way anova, tukey’s multiple comparisons test. (g) quantification of f-actin protein by elisa using head homogenates from young (10-day-old) and aged (30-day-old and 45-day-old, as indicated) canton s flies. N = 4 homogenates generated with 5 brains each per condition. *p = 0. 0192, **p = 0. 0053, unpaired two-tailed t-tests. H survival curves of canton s flies given a rich diet (5. 0% yeast extract) versus those undergoing dietary restriction (dr, 0. 5% yeast extract) from day 4 post eclosion onwards. ***p = 0. 001; log-rank test; n > 140 flies. I immunostaining of brains from canton s flies aged day 21 post eclosion provided a rich or restricted (dr) diet, as in (h), showing f-actin-rich rods (red channel, phalloidin). Scale bar is 5 µm. J quantification of f-actin-rich rods by phalloidin stain per 1 mm2 area of brains as shown in i. Dr n = 6, rich n = 5 flies, as indicated. *p = 0. 0225, unpaired two-tailed t-test. K survival curves of white dahomey flies given 10 µm rapamycin or vehicle from day 4 post eclosion onwards. ***p < 0. 0001; log-rank test; n > 250 flies per condition. L immunostaining of brains from young (10-day-old) and aged (45-day-old) white dahomey flies given 10 µm rapamycin or vehicle, showing f-actin-rich rods (red channel, phalloidin). Scale bar is 5 µm. M quantification of f-actin-rich rods by phalloidin stain per 1 mm2 area of brains as shown in l. D10 n = 6, d44 rapa – = 6, d44 rapa + = 9 flies, as indicated. **p = 0. 0023. , ***p = 0. 0001; one-way anova, tukey’s multiple comparisons test. Data are presented as scatter plots overlaying mean values +/- sem.
The Role of Dietary Restriction
Fruit flies on a calorie-restricted diet showed a marked decrease in F-actin buildup in their brains. Caloric restriction has long been associated with extended lifespan and improved healthspan, and this study provides additional evidence that it also reduces age-related protein accumulation, likely by enhancing autophagy.
Rapamycin as a Lifespan-Extending Drug
Rapamycin, an mTOR inhibitor known for its anti-aging properties, was also found to decrease F-actin levels in aged fruit fly brains. By inhibiting mTOR, a pathway that negatively regulates autophagy, rapamycin effectively promoted the clearance of cellular waste, thereby reducing F-actin accumulation and enhancing cognitive function.
Implications for Human Aging and Cognitive Health
While the study was conducted on fruit flies, the findings hold significant implications for human aging research. The buildup of F-actin in neurons and its impact on autophagy suggests a potential target for therapeutic interventions aimed at preventing cognitive decline.
Potential Challenges in Translating Findings to Humans
- Complexity of Human Brain: The human brain is far more complex than that of fruit flies, and the pathways regulating actin filament dynamics may differ significantly.
- Safety of Genetic Interventions: While gene editing techniques like CRISPR offer exciting possibilities, targeting actin-related genes in human neurons could have unintended consequences, given the protein’s critical role in many cellular functions.
- Pharmacological Approaches: Developing drugs that can safely reduce F-actin buildup without disrupting normal cellular activities remains a significant challenge.
Future Directions: Toward a Healthier Aging Process
The study’s findings open new avenues for research into how we can enhance healthspan, not just lifespan. By targeting the molecular mechanisms that contribute to cellular aging, particularly in the brain, we may be able to develop therapies that delay the onset of cognitive decline and improve overall quality of life as we age.
Research Goals Moving Forward
- Identify Additional Genetic Targets: Further research is needed to uncover other genes and pathways that regulate F-actin buildup and autophagy.
- Develop Safe and Effective Drugs: Pharmacological interventions that can mimic the effects seen in fruit flies, such as enhancing autophagy without significant side effects, are a priority for future studies.
- Clinical Trials in Humans: Translating these findings into human clinical trials will be crucial for determining the feasibility and safety of potential interventions aimed at preventing cognitive decline.
Conclusion: A Promising Path Toward Slowing Brain Aging
The buildup of F-actin in the brain emerges as a critical factor driving cognitive decline through its inhibition of autophagy. By addressing this accumulation through genetic, dietary, and pharmacological interventions, researchers have taken an important step toward uncovering the molecular underpinnings of aging. Although challenges remain in applying these findings to human health, the study offers a hopeful glimpse into strategies that could one day help us enjoy longer, healthier lives.
This comprehensive exploration of the role of F-actin in brain aging provides a new framework for understanding cognitive decline and offers actionable insights for developing interventions aimed at enhancing healthspan. The findings represent a significant leap forward in the quest to unravel the mysteries of aging and lay the groundwork for future research into healthier aging strategies.
Sources
- ScienceDaily – Scientists can reverse brain aging in fruit flies by preventing buildup of a common protein – October 28, 2024
- Nature Communications – Accumulation of F-actin drives brain aging and limits healthspan in Drosophila – October 25, 2024