Whether it is necessary to polish the abutments and the subgingival part of the crowns and what it gives in reality
The purpose of this article is to clarify the question of how the intra-gingival part of abutments and crowns should be treated. Whether they should be polished, or left at the machining level after drilling. This question has recently become very topical. The most popular recommendation is that polishing is necessary because it improves the epithelial attachment of the gingival cuff to the abutment. The tight connection between the epithelium and the abutment in itself prevents mucositis and peri-implantitis. As always, we will examine whether this is true, based on current research and practical observations of real patients.
Why polish abutments for implant crowns?
At first sight, the question does not seem to be important because dentures placed 10-15, and even 20 years, ago are still in successful use and there are no problems with gingival attachment, even though no one polished the abutments and subgingival parts of the crowns in those days.At the beginning of 2024, professional societies were bursting with questions and references to studies, not all of which were of good quality. Our task is to understand what is going on < and how surface structure affects:
- Epithelial attachment of the gingival cuff to the abutment;
- The development of pathogenic microflora that can attach to the surface of crowns and abutments.
Let’s start with the key differences between implants and superstructures that are used for prosthetics.
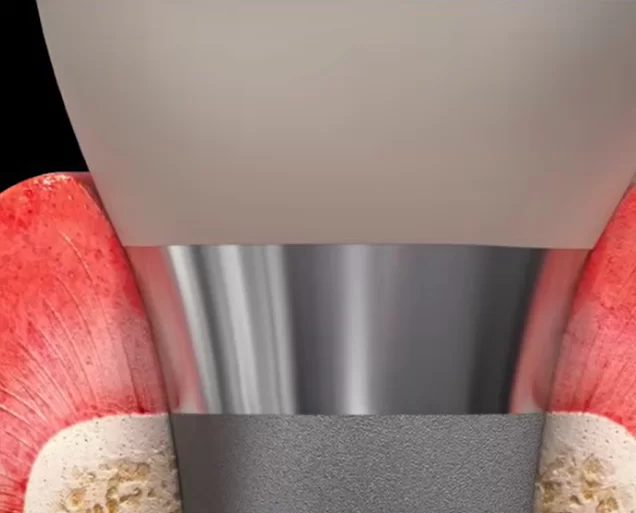
- Tissue-level implants are where the implant/abutment interface is at the level of the sulcus. The whole complex of soft tissue connection is formed around the implant cervix, which has a smooth, but not polished; part with a width of 1.8-2 mm not immersed in the bone, but is surrounded by soft tissue.
In this type of implant, the gingival cuff begins to form literally on the day of installation and will not be traumatised in any way. The soft tissue complex is formed just around the milled part of the implant. More accurately, the connective tissue connection is formed at the level of the rough part of the implant, and the epithelial complex, the physiological width of which is just about 2 mm, is formed around the milled part. There are no problems with this connection. One could conclude that milled titanium is a good enough surface and does not need polishing. However, another type of implant is used in most cases.
- Bone-level implants are where the implant/abutment interface is at bone level or even lower in the case of a subcrestal installation.
Here we see a very different picture, the gingival cuff is formed around the removable part of the restoration. First, it is the healing cap, then the permanent abutment with the crown. In the process of forming the gingival attachment, the soft tissue is repeatedly traumatised. Every time the healing cap is removed practicing dentists see a little bleeding. There is evidence that with frequent manipulations in this area, the soft tissue complex tends to sink lower so that the attachment is at the level of the fixed implant cervix. Because of this, it is possible to lose about 1 mm more of marginal bone around the implant.
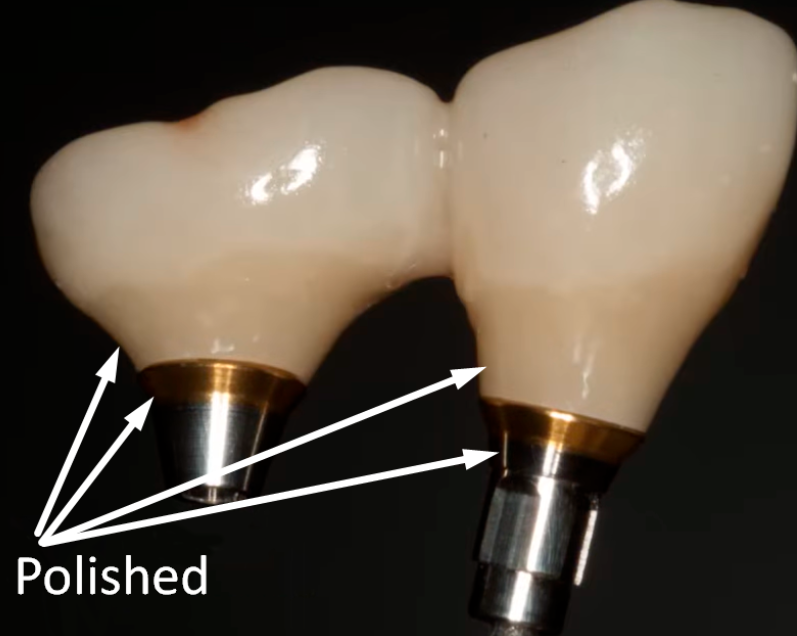
But back to our topic. It is the material from which abutments for bone-level implants are made and the degree of processing that is the subject of controversy and conflicting recommendations. Our task is to ascertain if polishing is necessary, what is the optimum degree of polishing, and what are the practical results for the patient. In the illustration below of a restoration supported on two implants, the areas that are recommended for polishing are shown.
Let’s formulate the problem – abutment polishing is recommended as a way of ensuring better attachment of epithelium to the abutment compared to milled surfaces. Until now there were no qualitative studies that confirm the mechanism of epithelial cell attachment in vivo. Neither is there any observed difference among practicing dentists. In general, epithelial attachment has proven to be as elusive as Father Christmas. It should be, as it should be. There are traces of its presence in the form of slight bleeding after removal of the restoration. However, not everyone bleeds and not always – does this mean that the epithelium simply contacted the surface and was not attached? However, the gingiva of such patients is healthy and there are no complications or complaints. Let’s try to sort this out.
Is there epithelial growth on abutments?
Finding a definite answer has not been easy. Many recommendations from different specialists and organisations refer to the same studies, which do not answer the question of how epithelial attachment occurs, or whether it occurs at all. Most articles on this topic have a common structure. Researchers took zirconium, titanium, and some other materials, made several samples with different degrees of surface treatment and grew epithelial cells or some species of bacteria on their surface, and made profound conclusions that this or that variant is better suited for practical application. The in vitro result cannot, however, serve as a full-fledged basis for practical recommendations.
Moreover, the practitioners among our readers will confirm that the restoration comes out of the gingival socket very easily. Even the very “honey stickiness between the fingers”, which Dr. Carl E. Mish wrote about, cannot be felt (this refers to the fact that the epithelial cells are attached to the surface through polysaccharides and must be detached with a slight force comparable to fingers sticking to a drop of honey).
The first study we will look at investigated the question of the attachment of living cells to titanium in general. It took place in vitro, which means that it was necessary to grow cells on the surface and evaluate whether attachment occurred.
The bacterium Escherichia coli (E. coli) was taken as a model organism because this bacterium attaches to surfaces according to the same principles as epithelial cells (epitheliocytes).
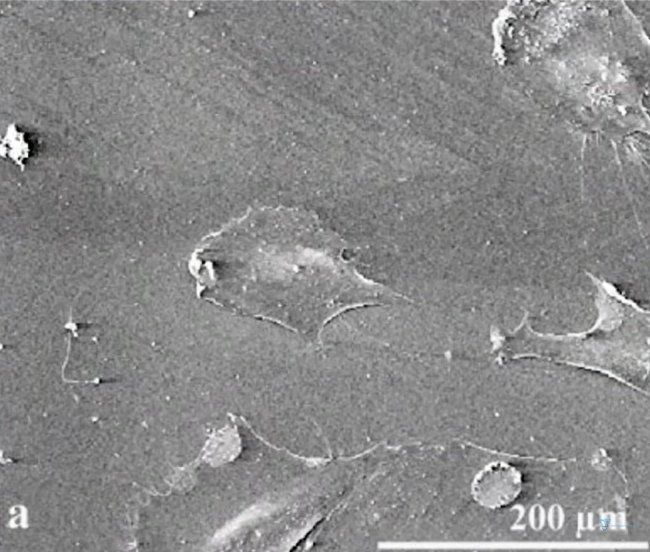
To detect the connection, the protein vinculin, which is always present in the attachment of a living cell, was observed. If we evaluate its presence and amount, we can say that the connection of a living cell to the surface has occurred. The image below shows the visualisation of the presence of vinculin at the point of contact between the living cell and the titanium surface.
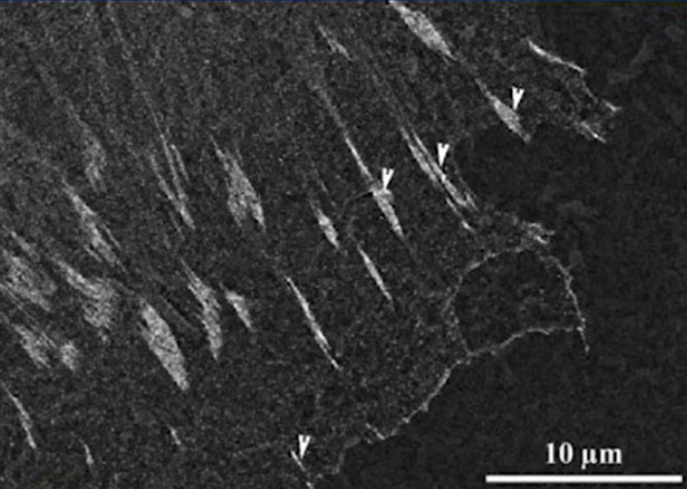
Immunogold staining and detection of vinculin in focal adhesions using backscattered electron (BSE) imaging on SEM. BSE imaging of immunogold staining of vinculin in focal adhesions in E-cell on P surface Focal adhesions 48 hours after seeding are marked with arrows
This study is the basic one, and many people refer to it and conduct their studies on the same principle. Nowadays it is not a problem to get any cell culture in unlimited quantities. Indeed, in conditional petri dishes, something grows and attaches. How much these results are apply to practice is a big question.
Next, let’s consider the work of Professor Maria Welander’s team from 2008. This work is interesting because it was carried out on animals.
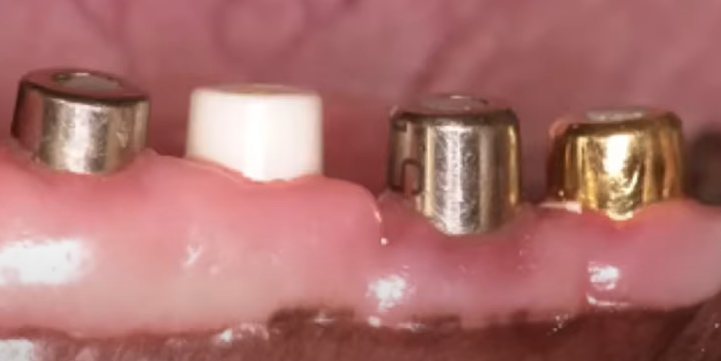
Dogs were implanted with abutment implants made of different materials, including zirconium, gold and titanium.
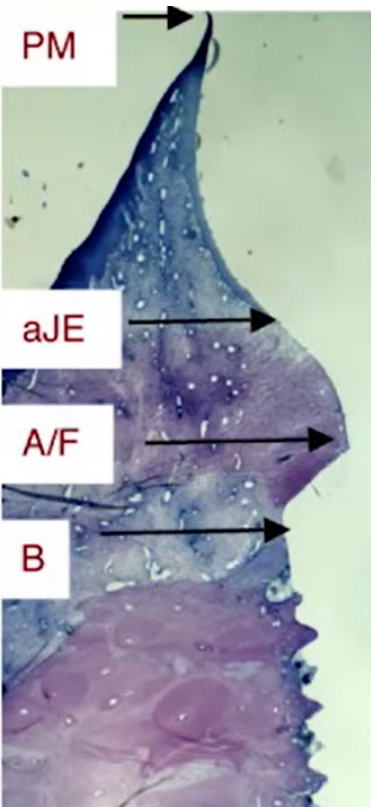
After some time, namely 2, and 5 months, histological analysis of the tissues in the area of attachment was done. All data are qualitatively documented and illustrated. In the sections, we can see the tissue distribution familiar from previous articles on the topic of soft tissue integration. The quality of these tissues can also be assessed from the histological results.
Tissue quality refers to the stability and immobility of this soft tissue ring. It is the stability and immobility of the gingival cuff that provides high resistance to various peri-implantation diseases, especially mucositis and peri-implantitis. It is this resistance we want to achieve. The problem is that within the context of this study, it was not shown that the epithelium was attached to the material in any way. Yes, the histological data clearly show keratinized gingiva, you can see where it ends, and how the soft mucosa is further formed into sulcus. However, no significant difference was found between the epithelium in the well that was in contact with the test materials and the normal oral mucosa.
According to the results of this study, it is not clear whether the epithelium connects with the test material, whether there are focal adhesions, or whether the epithelium simply adheres to the surface and is held by the elasticity of the gingival cuff.
In addition to the studies mentioned above, you can find many publications that refer to the in vitro studies we have already mentioned, or even refer to each other. The most “fashionable” conclusion is that the best material for abutments is zirconia, and it should be polished. These conclusions, however, do not follow from the results of studies that refer to the authors of publications.
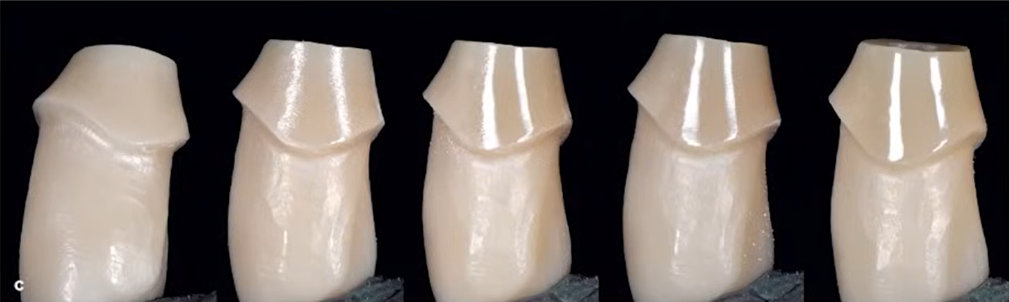
Our task is to get to the truth, which will not be so easy. For example, let’s turn to the book “Zero Bone Loss” by Dr Thomas Linkevicius, which is undeservedly little known in the USA. There is a whole chapter on how to work with the intra-gingival part of the restoration. This author strongly recommends polishing the intra-gingival part. The change in surface condition during zirconia polishing is shown in the image below.
Apart from the author’s passion and enthusiasm, the book also lacks objective data on why the surface should be polished. Looking ahead, it is true that a polished zirconia surface does have advantages over an unpolished one, but not at all those cited by the authors of the above-mentioned studies.
The interesting thing is that if you look deeper, you will find many studies with contradictory results.
Some claim that milled titanium is better than polished zirconia. Others state that titanium is inferior to zirconia with any degree of surface finish. There is also evidence that milled zirconia is already sufficiently prepared for soft tissue integration. This opinion has been dominant for a long time because there are several authoritative papers that confirmed it.
Key differences between milled and polished surfaces
Our search for the truth would be incomplete if we did not clearly understand the difference in the structure of polished and milled surfaces. After all, to the naked eye, both options look smooth enough.
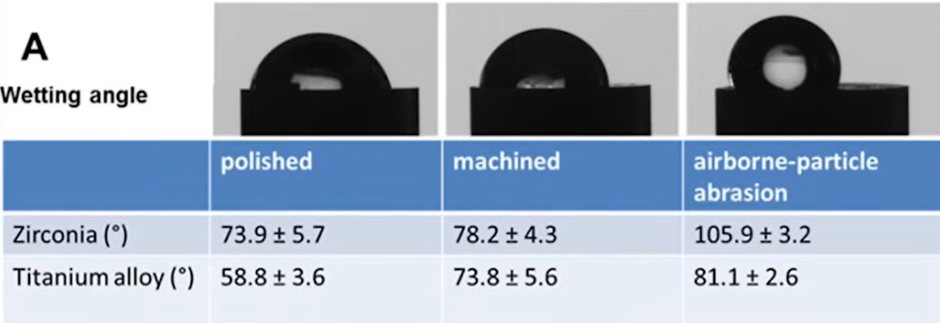
One of the evaluation methods is the wettability test, that is, the determination of the shape of a drop of water that is located on the surface. In the table below we can see that the drop has the flattest angle on the polished surface, with the titanium surface outperforming the zirconia surface in this measure. Three variants are shown here, and if the difference between the polished and milled surface is not so critical, on the abrasive-treated surface the drop angle is greater than 90°, i.e. the drop starts to turn into a ball.
Although this is an indirect and not very accurate method, it is a good way to determine the physical properties of a material. After all, with the same topology, different materials interact differently with biological fluids and living cells.
There are objective methods of determining surface roughness using a microscope, where the difference between peaks and troughs is simply determined. After all, even the most perfectly smooth surface under the microscope still looks like a series of irregularities.
It is generally accepted that:
- After milling, the average height of irregularities is 0.2 µm; and
- A polished surface is one where the height of irregularities is less than 0.1 µm;
What else besides the epithelium is attached to the crown surface and the subgingival part of the abutment?
Not all researchers specify the degree of roughness of their samples. Therefore, the results of the following study from colleagues in Japan are particularly valuable to us.
They prepared several material samples with different degrees of processing: rough, milled, and polished. Among the test materials were: titanium, zirconium, precious alloys (Au-Pt), aluminium oxide, and even hydroxyapatite.
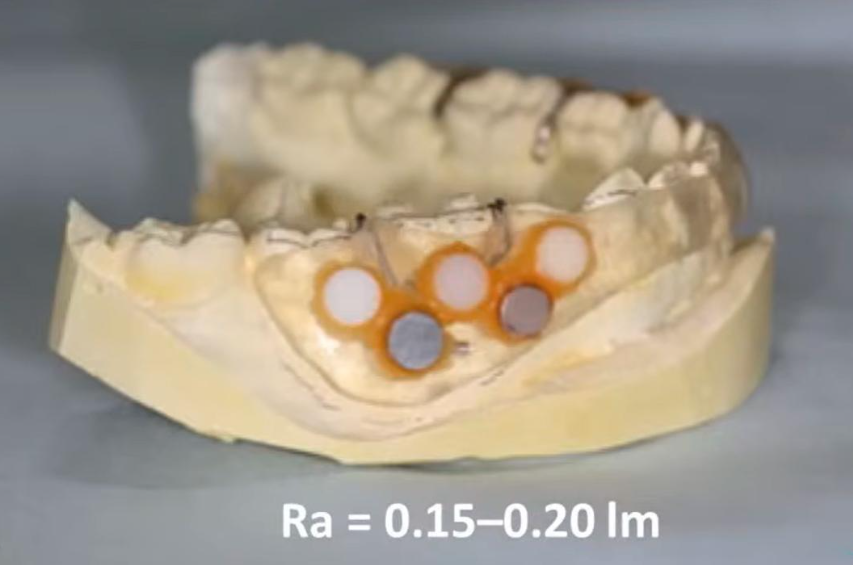
Test samples of materials that volunteers wore in their mouths to see what microflora would grow on the surface
Volunteers wore a design like the one pictured above for about a day (26 hours on average). Then this design was removed, and the surface was examined to see what bacteria grow on each of the surfaces made of different materials, and in what quantities.
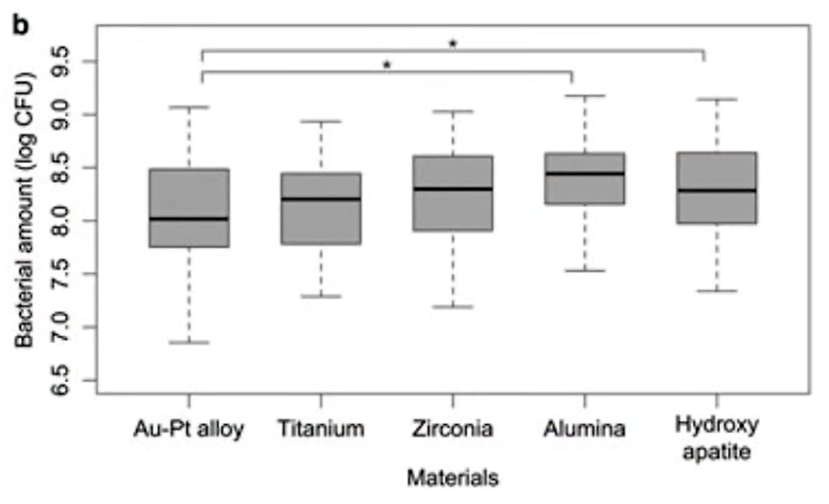
We are interested in data on polished surfaces, so we took a variant with roughness at the level of 0.15-0.2 microns – this almost equals the generally accepted indicator of a polished surface. After the assessment of the number and composition of microbiota was made, the experts found the following pattern, see the graph below.
- The polished alloy of gold and platinum showed very good results and was the most hygienic.
- Zirconium also showed good results.
- Titanium, on the other hand, did not perform well. The group of subjects was divided into two subgroups, some had much better results than others. This is what the researchers attempted to explain. Titanium is known to inhibit bacterial growth under aerobic but not anaerobic conditions (Bundy et al. 1980). This study was conducted under aerobic conditions, but a significant layer of plaque on the surface could have created an anaerobic environment, and the results of the study could have been affected by the difference in relative aeration between patients. It is also important to consider the fact that biofilms in peri-implantitis consist mainly of anaerobic bacteria (Koyanagi et al., 2010). Thus, the objectivity of the study is limited by the fact that it was conducted under aerobic conditions.
Therefore, the study’s conclusions about the degree of hygiene of different materials were ambiguous concerning titanium:
“In conclusion, the types of bacteria attached to the discs were similar among the materials tested but varied significantly between the study subjects (materials)”.
“The Au-Pt alloy accumulated less plaque than the other materials tested. However, plaque accumulation on titanium varied between subjects, which divided the group into two subgroups (high and low accumulation). The reason for this phenomenon requires further investigation.”
In the context of our article, the most interesting fact is that the same surface may well support both epithelial attachment and pathogen growth.
How cells attach to different materials
Consider another interesting study by specialists from Switzerland. They, too, did a similar split with different materials.
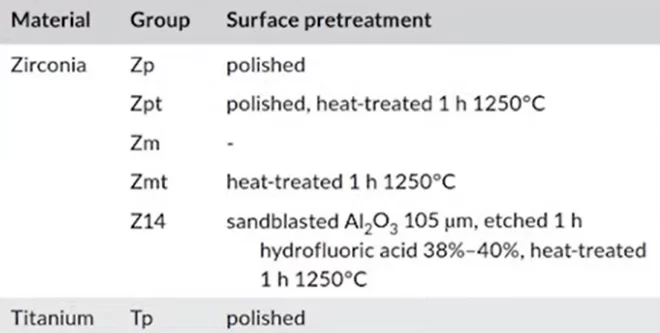
The questionable point is that only one sample of polished titanium was involved in the comparison. Whereas zirconium was presented in many variants: polished, milled, abrasive treated ( blasted), baked, etc.
Nevertheless, this is a qualitative study in which the scientists discovered how test microorganisms grow on, and attach to, different samples.
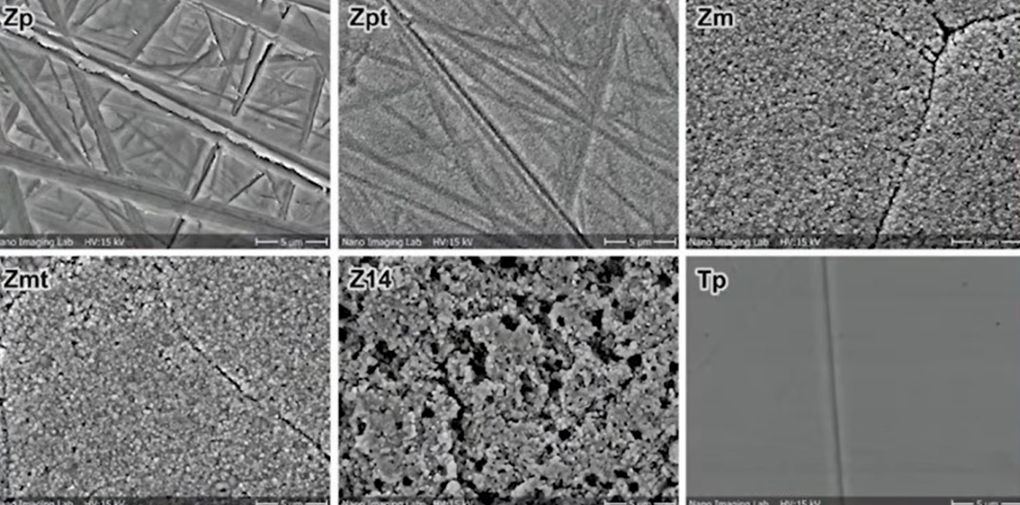
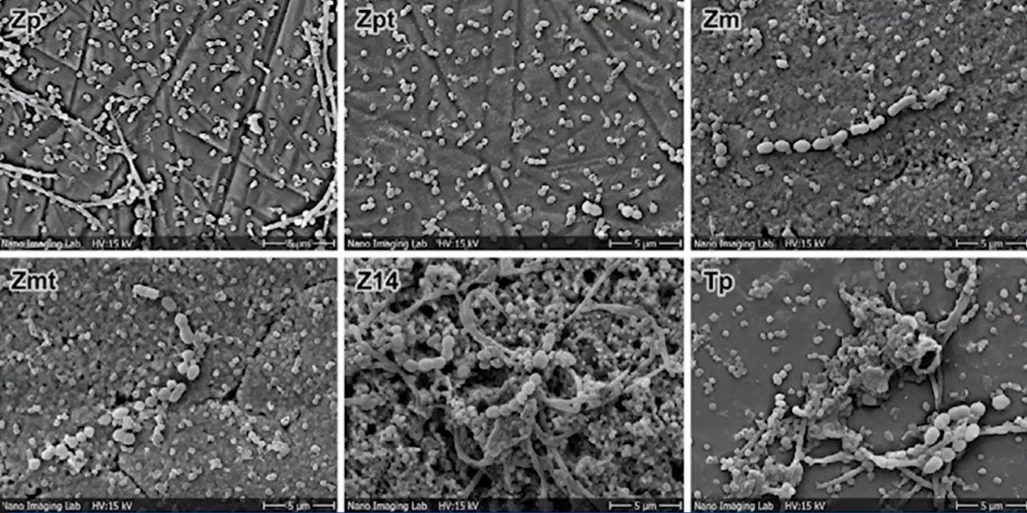
The test microorganisms were: Porphyromonas gingivalis, and Streptococcus sanguinis. The results of the seeding after 72 hours can be clearly seen in the pictures below.
As can be seen from the images, bacteria grew on all samples. However, as can be seen on slide Z14, where a very rough surface was created, the bacterial content is several times higher compared to the other samples. The least amount of microorganisms were on the polished surfaces of zirconium, and titanium.
Study Conclusion:
Surface roughness of Ra > 0.3 μm was demonstrated to be associated with increased biofilm formation. There were no statistically significant differences between polished (Ra = 0.1 µm) and machined zirconia surfaces (Ra = 0.3 µm). The tendency for increased biofilm formation was associated with increased roughness.
This corresponds to the threshold value for dental materials of Ra < 0.2 μm, which has previously been reported to prevent plaque formation (Bollen et al. 1997g).
That is, to reduce the amount of biofilm you need a surface with an average roughness < 0.2 µm, which is almost the same as a polished surface. This is known to all practising dentists who polish fillings to a so-called “dry shine” because if this is not done, plaque with many pathogens will accumulate on the surface.
A look at the problem of epithelial attachment from the perspective of science and practice
However, the previous study was about biofilm, not about epithelial attachment, although there are many similarities in the attachment of bacteria and epithelial cells. We are interested in finding a study that will test how epithelial attachment to the abutment occurs. A good candidate for such a study is the work of Japanese specialists, and it is quite recent from 2019.
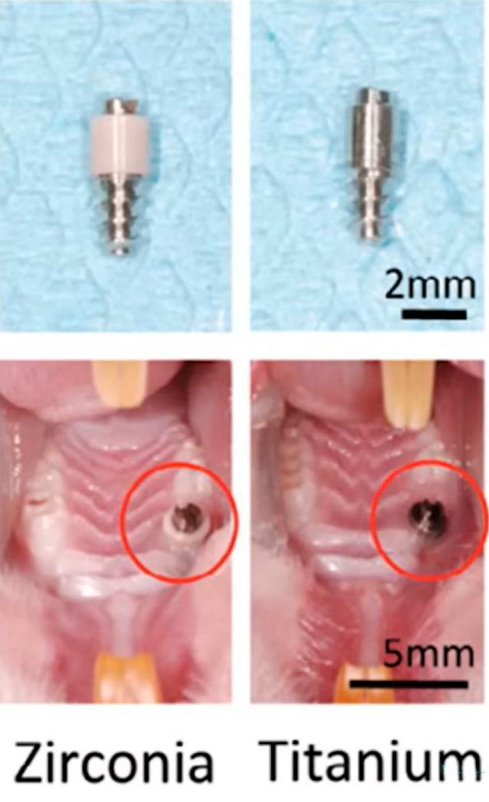
Special implants were made for the study, see image below. A zirconium rod passes through the soft tissue in the first case, and a titanium rod in the second.
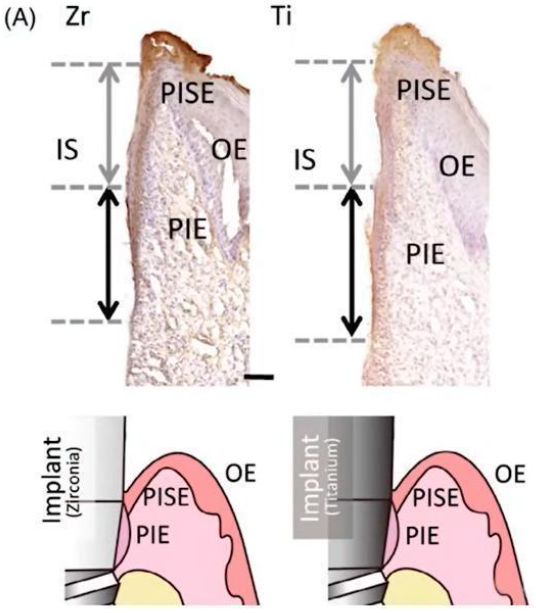
Tissue distribution of different types of epithelium in contact with titanium and zirconium surfaces
Here are the results they published:
“We compared the effect of materials on the sealing function of the peri-implant epithelium with zirconium dioxide and titanium dioxide implants for clinical applications.”
Conclusions:
Zirconium dioxide suprastructure has a more aesthetic view, which is important for modern approaches in implant treatment. However, the peri-implant epithelium around zirconia implants may be imperfect, as zirconia implants showed poorer epithelial cell adhesion compared to titanium implants.
This conclusion is in complete agreement with practical observations. The already mentioned tissu-level implants have been used for many years, and there are rarely any problems with epithelial attachment. The gingival cuff is stable and airtight and is formed around milled titanium. Yes, we still do not fully understand all the chemical and physical processes that accompany such an attachment, but the practical results are clear.
Our review would not be complete without examples of strange and controversial studies. For example, this paper from 2023.
The study aimed analyse the data and determine which surface finish of titanium, and zirconium, is best suited for epithelial attachment so that the gingival cuff is stable and rigid. This is what is needed to minimise the risk of various diseases.
The study is well described and contains many illustrations, but again it does not answer how epithelial attachment occurs. There are references in the study to earlier data by Linde and Abramson, but nothing new was learnt.
These are the conclusions the research team reached:
Meanwhile, there is a clear lack of basic/modern scientific evidence, adapted to the clinical market, regarding innovative trans-gingival platforms for dental implants.
Most of the surface modification technologies described in this critical review are still not available on the dental market and this shows the need for future transfer of basic research into commercial products.
This is a worrying sign. Logically, it can be assumed that the research group is asking for money in this veiled way, and is ready to issue recommendations that a product treated in this way is better than other options. This violates the main rules of scientific enquiry – impartiality and objectivity. Of course, we are in no way accusing this research group of anything, but cases in which scientific data were adjusted to suit the interests of commercial companies are not unknown.
And still – to polish or not to polish zirconia and titanium dioxide abutments?
Let us summarise the interim results.
- The recommendation to polish the subgingival portion of crowns and implants is reasonable and has practical benefits, at least from a hygienic point of view. It has been found that biofilm formation is much less on polished surfaces compared to rough surfaces.
- A tight and airtight cuff around the restoration is a major factor in reducing the risks of inflammation. The condition of the gingival cuff around the polished trans-gingival portion of the restoration is better compared to porous and rough surfaces. Even if the mechanism of epithelial attachment is not fully understood, it cannot be reliably ascertained whether there is any attachment at all.
- In addition to polishing the abutments and subgingival parts of the restoration, these surfaces must be thoroughly sterilised and cleaned of silicone micro-fragments and other contaminants that impair epithelial contact with the surface. Cleaning can be carried out either simply with alcohol or in an ultrasonic bath with alcohol or other special solutions. Steam treatment and autoclaving, as well as a combination of several cleaning methods, also give good results.
We recommend Dr Tarnow’s book. Even experienced specialists will find there a lot of useful and well- systematised information and practical recommendations.
In addition to being a great clinician, Dr Tarnow is also a talented scientist. A group of researchers under his leadership did some interesting work published in 2016.
These are their published conclusions:
Bleeding on initial detachment of the temporary restoration was significantly correlated with less change or greater stability in cheek ridge size at each control point from 0 to 3 mm apical to the free gingival margin.
Explanation. After implant installation, a healing cap or a temporary composite restoration was placed. Scientists measured the parameters of soft tissue and bone at this stage. They measured all the parameters again 3-4 months after the removal of the healing cap or temporary crown. It transpired that in those cases when after the removal of the construction there was slight bleeding, the condition of soft tissue and bone was much better compared to cases when there was no bleeding.
To better understand what this may be related to, let’s consider an example from Dr Tarnow’s book mentioned above. There, a patient was fitted with a customised healing cap made of composite material. It was then removed by cutting out a fragment of the gingiva adjacent to the healing cap.
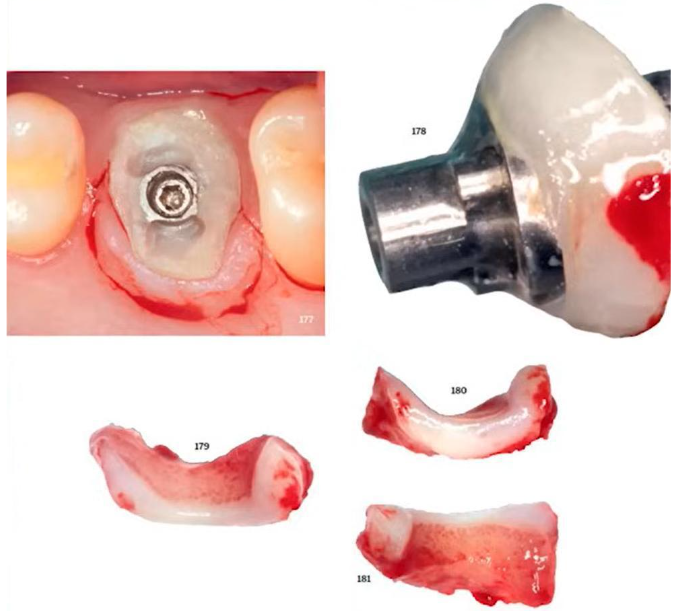
Experiments from Dr Tarnow’s book – study of the tissues that were in contact with the composite healing cap
These tissues were examined carefully, and the conclusion was:
Clinically, fibroblasts can adhere to the surface of the restorative material provided that the surface is clean and microporous, allowing the cells to physically “stick”.
That is an interesting conclusion. All previous research and studies that we have analysed were aimed at proving how a polished surface is important for epithelial attachment. It transpires that the attachment is to a rough surface. This experience was conducted by a practising clinician under real human conditions.
Furthermore, Dr Tarnow also has in vitro experiments, here’s a study from 2019.
The work was focused on manufacturing customised healing caps, so composite material was used as the test material. Several surface variants were prepared:
- Polished;
- Unpolished microporous;
A thorough cleaning of the surface was a prerequisite. They found that vapour blasting and ultrasonic washing with alcohol worked best.
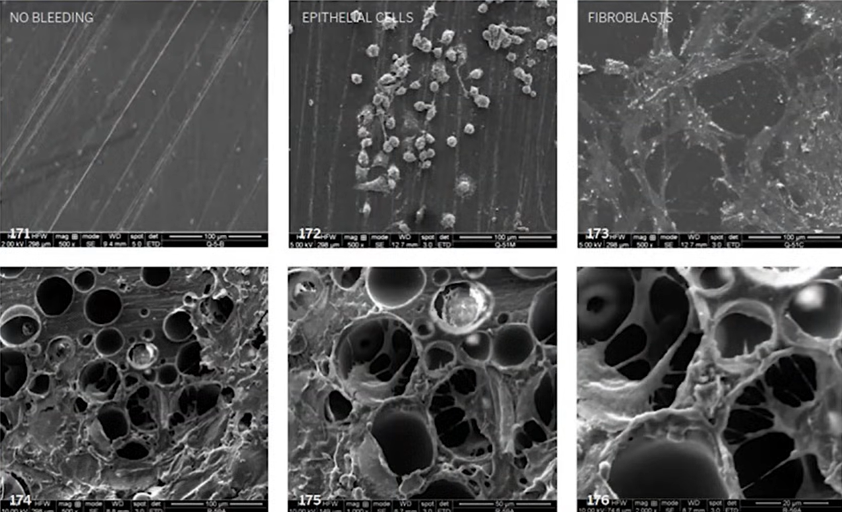
The scientists then observed fibroblasts and epithelial cells growing on these surfaces, see the pictures below.
It was found that there was no cell growth on smooth surfaces. On rough surfaces, epithelial cells and fibroblasts grew well. This explains why there is no bleeding when removing polished trans-gingival elements. It means that epithelial attachment is not occurring.
The unexpected conclusion is that it is not necessary to polish the abutments to obtain epithelial attachment. On the contrary, the fewer irregularities there are, the less chance that cells will attach.
It’s not that simple, Dr Tarnow’s research focused on the selection of materials and their degree of processing for temporary structures. It is the microporous structure of the composite material that allows the volume and level of the gingiva to be maintained during the healing stage. At this stage, the epithelium grows against the healing cap, and a resilient and high-quality gingival cuff is formed. Furthermore, Dr Tarnow does not suggest making permanent abutments from composites; we already know that a surface that is good for the growth of epithelial cells is also good for the growth of pathogenic microflora. Therefore, a microporous structure is good for the formation of the gingival cuff, but not for a permanent restoration. If the gingiva has a good contour and is dense and elastic, then when installing a permanent denture with a titanium or zirconium abutment, the tightness ensures that the epithelium adheres tightly to the polished surface. Whether there is epithelial adherence is less important. It is more important that the polished surface is more hygienic and, therefore, the risk of mucositis and peri-implantitis is reduced even if there is no epithelial connection.
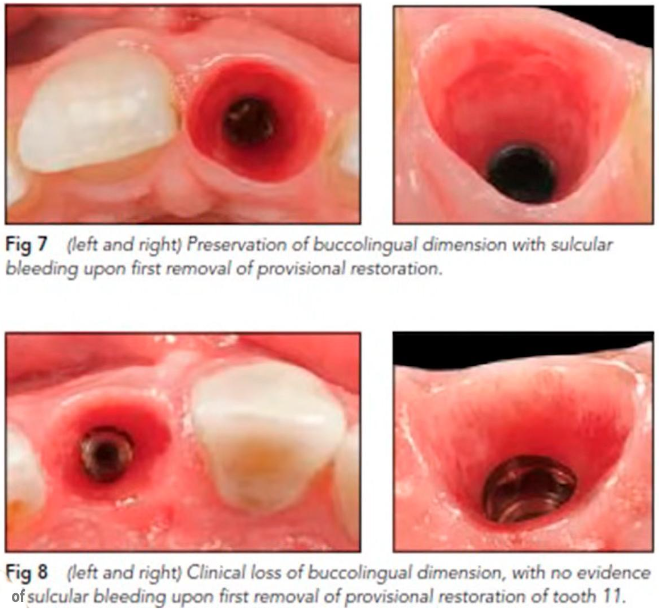
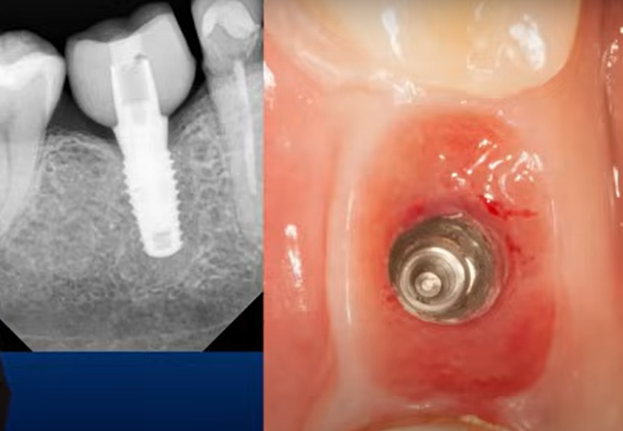
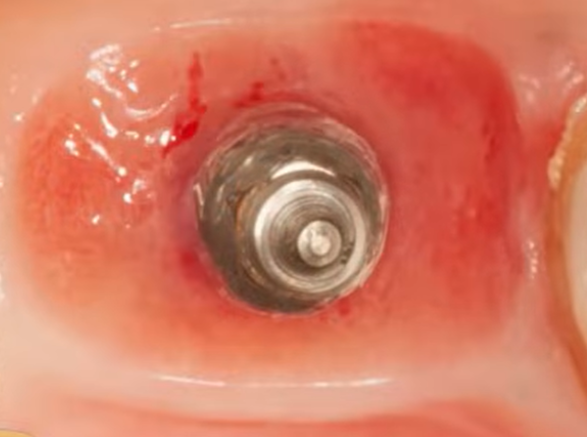
This conclusion is supported by practice, see the images of one clinical case. A single implant was placed, healing and integration occurred without complications. The abutment and the subgingival portion of the crown were polished and thoroughly cleaned. Three years after the installation of the restoration, the crown needed to be replaced. X-rays showed good bone health. The gingiva was also in good condition, but note the condition of the well after removal of the abutment.
On a larger magnification, you can see that the inner surface of the hole is shiny and there’s almost no bleeding. The shiny smooth and healthy epithelium, with a high content of keratin, simply came into contact with the trans-gingival part of restoration. The attachment, if any, is closer to the interface line of the implant/abutment, as indicated by slight blemishes. So, we have a fairly successful restoration, but almost no epithelial attachment.
This is what practitioners most often see, which is why there is no sensation of adhesion of the restoration to the epithelium.
Let us now reflect on the data obtained, and summarize the results. On the one hand, we need a microporous surface for the epithelium to grow. On the other hand, we know that bacteria grow well on rough and porous surfaces, the bacterium Escherichia coli was even used in some studies as a test microorganism.
To summarize, we agree that both titanium and zirconium abutments should be polished, but for a completely different reason. A hygienic and easily cleanable surface is much more important than epithelial attachment.
The second conclusion that can be drawn is that the degree of machining is sufficient. Most studies confirm that milled titanium is still the gold standard. There is little difference between polished and milled titanium in terms of hygiene and cell attachment.
Practical conclusions
- The surface does not have to be smooth for good epithelial growth, so healing caps made of composite material with a microporous surface work well during the healing phase.
- The trans-gingival portion of the permanent restoration should be smooth. How smooth is still under discussion, but today the optimal surface is milled titanium. This is confirmed by the success of tissue-level implants, which contact the epithelium precisely with the milled part.
- Polishing improves the hygienic properties of the surface, although only to a moderate extent. Therefore, polishing the trans-gingival portions of the restoration can be recommended for all bone -evel implant restorations.
If you have any questions about this topic, please feel free to leave them in the comments.