Cement retained Zironium Bridge with Custom Titanium Abutments. Implant-Supported Restoration. 67 Year Old Male with an Uncomplicated Medical History

Abstract
The patient presented with missing teeth (#3 and #5) and had an unsuccessful partial denture. Implant placement was recommended. A PA X-ray confirmed implants were viable, and the procedure was scheduled with bone grafting, PRF, and guided tissue regeneration for optimal success. During the implant placement, PRF was prepared through centrifugation, and local anesthesia was administered to ensure patient comfort. A full-thickness mucoperiosteal flap was created, and osteotomies were prepared using Densah drills. Implants were placed with careful positioning and covered with PRF membranes. Healing caps were placed after three months, and the tissue was allowed to heal for an additional three weeks. The final impressions were taken using both open and closed tray techniques, with PVS material ensuring accuracy. The healing caps were cleaned and repositioned, and the shade (A3) for the restoration was recorded. The patient’s implant-supported zirconia bridge was delivered, ensuring proper occlusion and fit. The bridge was cemented with Panavia SA dual cure cement, and flossing instructions were provided. The patient was very satisfied with the aesthetic outcome, and a final PA radiograph was taken to confirm proper placement.
Download Case Study (PDF, 866 KB)
The value of this case for the dental Community
Demonstrates a comprehensive approach to implant placement, integrating PRF, guided tissue regeneration, and careful surgical planning. Highlights the importance of proper osteotomy preparation and implant positioning for long-term success. Provides insight into handling soft tissue management and achieving optimal healing for better prosthetic outcomes. Reinforces the significance of precision in final impressions and occlusal adjustments to ensure patient satisfaction. Emphasizes the role of patient education and postoperative care in maintaining implant longevity and overall oral health
Patient History
67 year old male patient arrived to discuss implant options for their upper right side. They have already teeth #3 and #5 extracted at another practice. They also had a cast partial denture fabricated, but unfortunately, it proved to be unwearable.
Clinical Examination
A PA was taken, confirming that, as previously planned, implants are indeed a viable solution for this area.
Diagnosis
Patient with uncomplicated medical history.
Treatment Implementation
4/23/24
Patient Consultation
The patient arrived today to discuss implant options for their upper right side. They have already teeth #3 and #5 extracted at another practice. They also had a cast partial denture fabricated, but unfortunately, it proved to be unwearable. The potential pitfalls of combining a partial denture with clasps that place stress on implants were discussed, as this could ultimately jeopardize the implants long-term success. A PA was taken, confirming that, as previously planned, implants are indeed a viable solution for this area. With the patient expressing their readiness, the implant procedure was scheduled for approximately four weeks out. The plan was to have implants placed at the #3 and #5 sites, utilizing bone grafting, PRF, and guided tissue regeneration to ensure the best possible outcome.
5/21/24
Implant Placement
Before getting started, a phlebotomy was performed, and three red 9ml vials and one white 9ml vial of the patient’s blood were drawn from their left arm’s antecubital region. This was the beginning of PRF preparation. The vials were then placed into the centrifuge and processed following precise protocols. First, the I-PRF process was employed, spinning the vials at 3200 rpm for 3 minutes. This allowed the top separation layer to be drawn off, mixed with 1.0cc allograft (ZGraft-mineralized cortical/cancellous bone particulate), and left to oxidize for at least 20 minutes, resulting in “sticky bone.”Next came the A-PRF process. Here, the tubes were spun at 2700 rpm for 8 minutes. After the centrifuge, the tubes were uncapped and allowed to oxidize for 5-10 minutes. The fibrin clots from the red tubes were then carefully extracted and placed into the PRF processing box for the creation of A-PRF membranes. It was decided to have implants placed at sites #3 and #5. The patient had been pre-medicated with antibiotics the
night prior, and informed consent was thoroughly reviewed and obtained today.
Local anesthetics were then administered:
• one 1.8ml carpule of 2% lidocaine with 1:100K epinephrine
• three 1.8ml carpules of 4% articaine/septocaine with 1:100K epinephrine
• 1.8 ml carpule of 0.5% Bupivicaine with 1:200k epi.
This ensured the procedure would be as comfortable as possible.
A pre-operative X-ray of sites #3-5 was taken, confirming that everything was ready. Two carpules of 2% lidocaine with 1:100K epinephrine were then administered via infiltration. A full thickness mucoperiosteal flap was prepared with crestal incision from mesial of #2 through distal of #6 site. Subsequently, vertical releasing incisions were prepared. The implant osteotomies were initiated with a 2.0mm drill at 900rpm, using copious amounts of sterile saline water irrigation. This ensured good visibility and kept things cool. Drilling to a depth of approximately 7mm took place, and then parallel guide pins were placed to verify positioning with another PA radiograph.
A 1.8mm Lindamann bur was utilized to correct the positioning of #3 osteotomy. The osteotomies were then finalized with Densah drills in reverse rotation to 11mm at site #3 and 12mm at site #5.The osteotomies were then finalized with Densah drills in reverse rotation to 10mm at site #3 and 12mm at site #5.
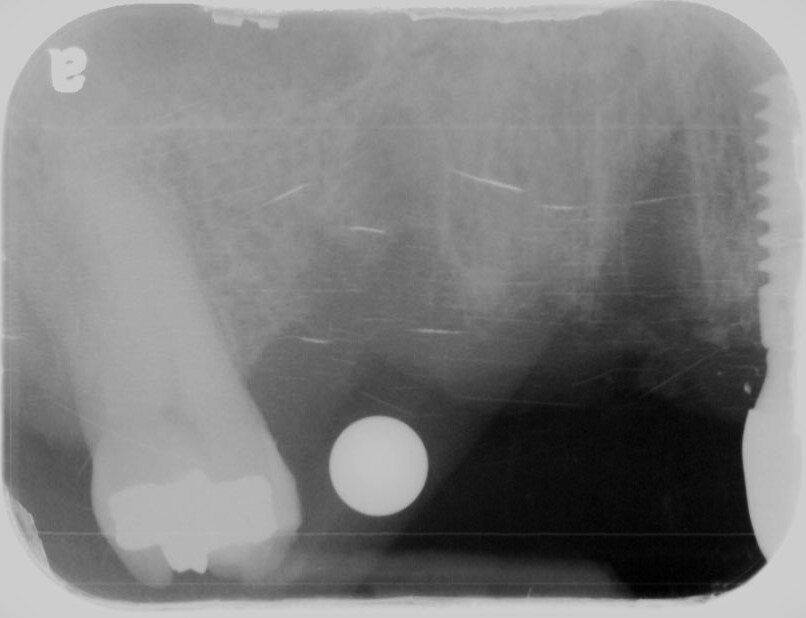
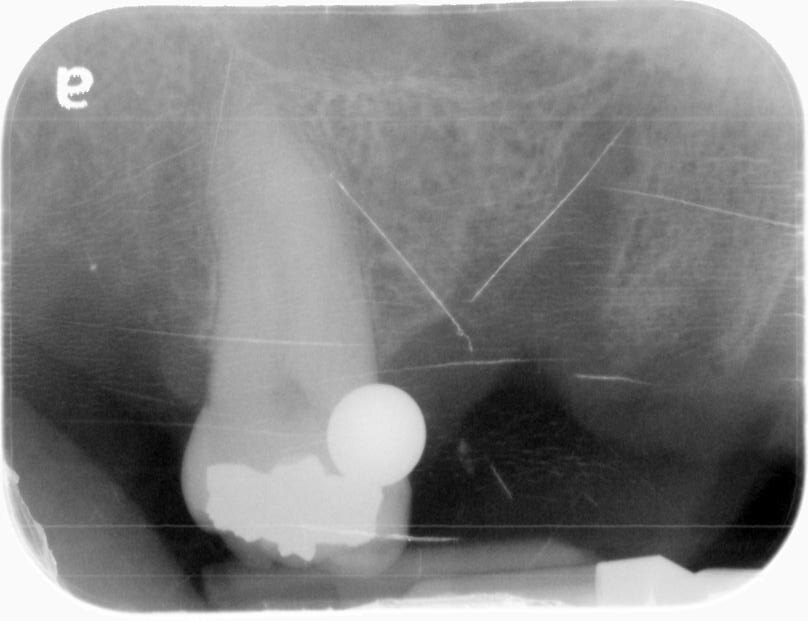
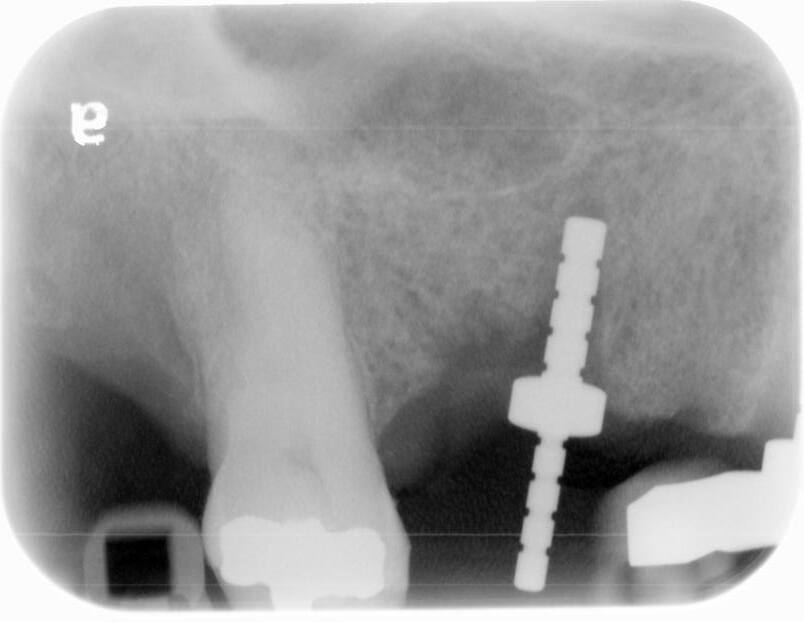
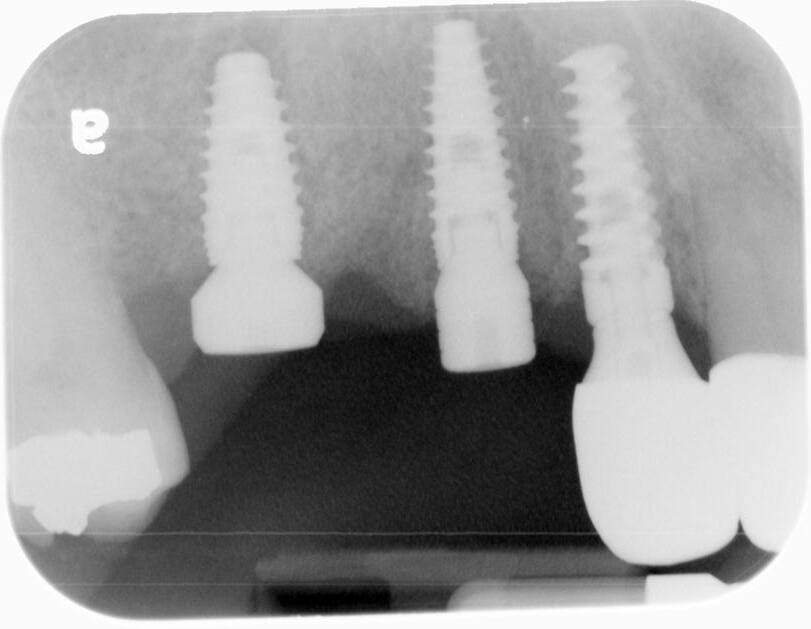
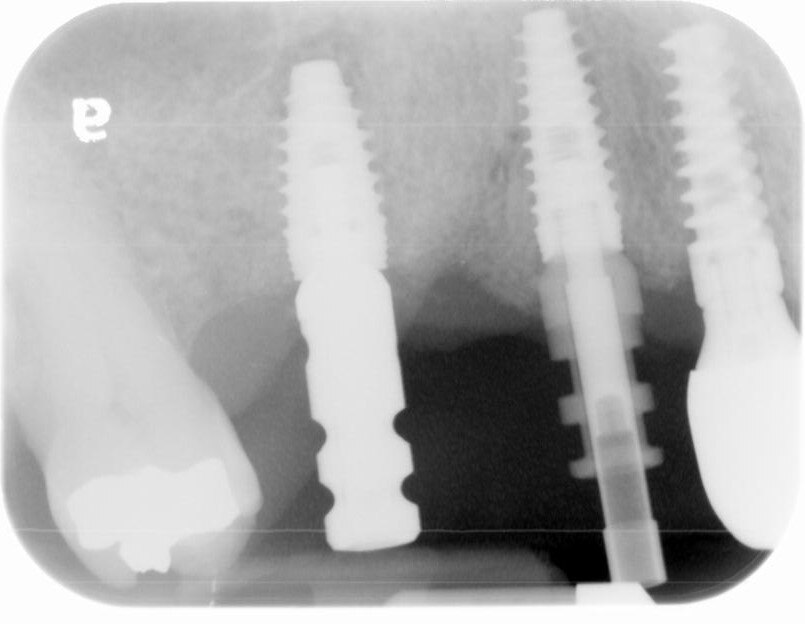
A UH8 implant, measuring 5.0 x 10mm, was placed at site #5, and a 4.2 x 11.5mm UH8 implant was placed at site #3, at 30rpm and 40Ncm. Both implants were placed approximately 1mm subcrestally. Cover screws were secured, and a post-placement PA radiograph was taken to ensure all was in perfect alignment.
Then, the “sticky bone,” crafted from ZGraft 70/30 cortical mineralized/demineralized blend mixed with the patient’s blood/PRF and sterile saline, was placed into the voids. A resorbable collagen membrane was carefully trimmed to extend 2-3mm over the labial and lingual bony crests, and then covered with PRF membranes. While primary closure was not fully achieved, the membrane and graft were secured using 4.0 PTFE horizontal mattress and interrupted sutures. The postoperative instructions were gone over in detail, both verbally and in writing, and the patient tolerated the entire procedure well.
Biologics: ZGraft-min.cort/can.bone part, resorbable collagen membranes, and PRF
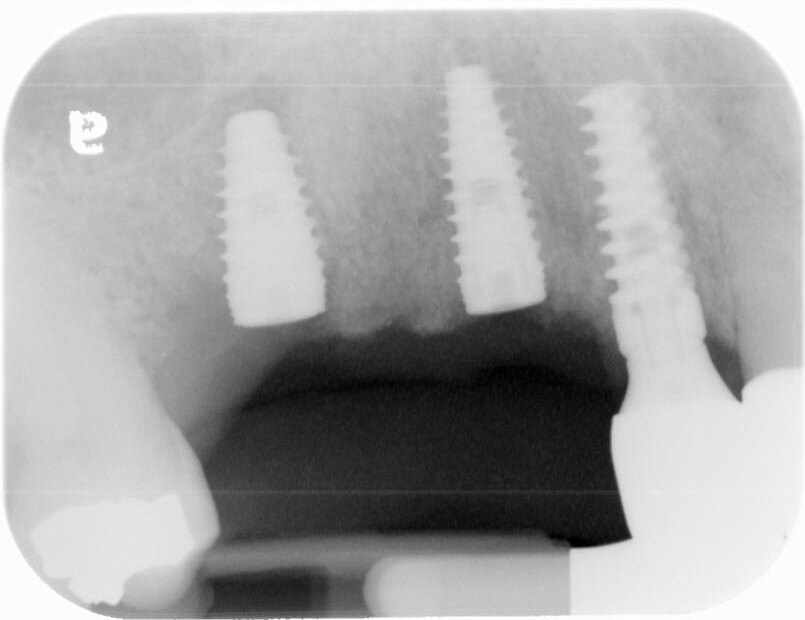
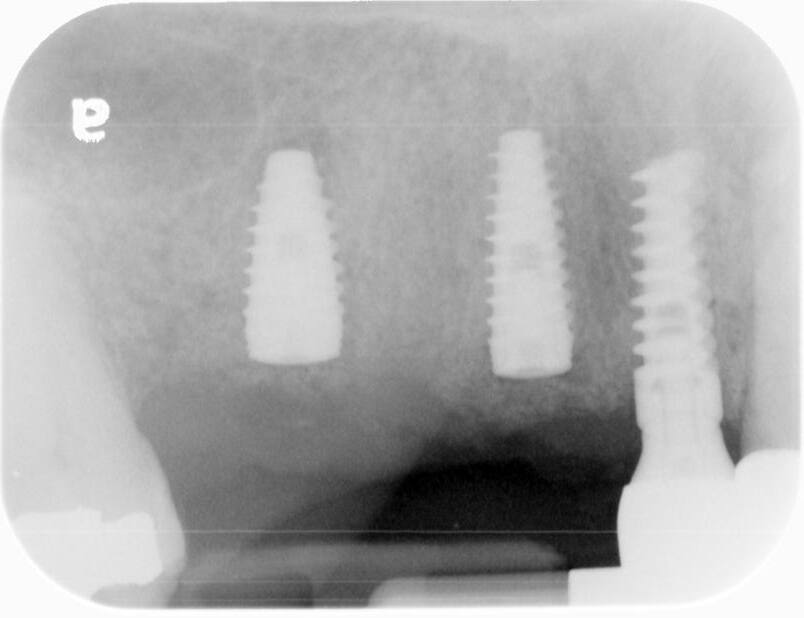
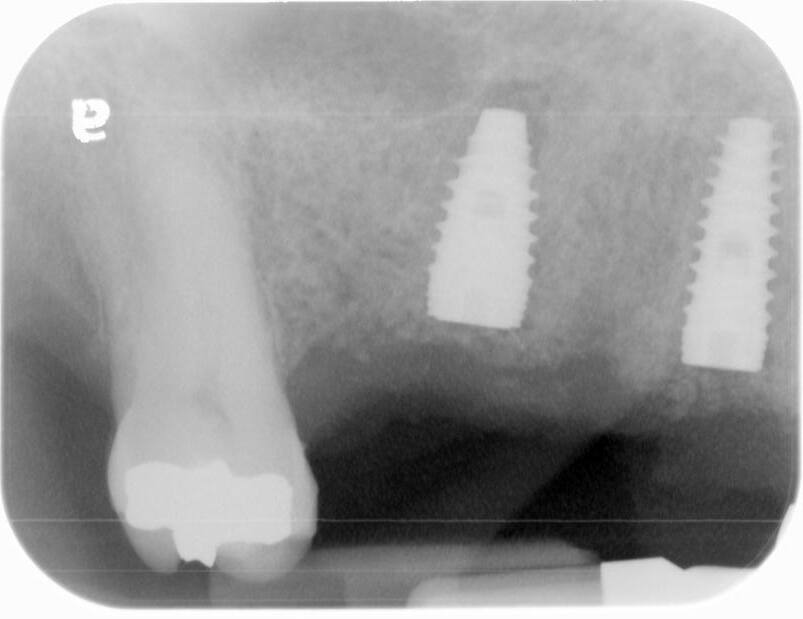
The post-operative check and suture removal were scheduled for 2-3 weeks down the line.
7/30/24
Healing caps placement at #3+5 sites
NM changes. Oral health of the patient was good. It was decided to place healing caps at #3+5 sites. One carp. of 4% septocaine was used as local anesthesia. Slow speed motor driven hole punches to expose the buried Uniqa Implants. Cover screws were removed. Uniqa 5.5 X3 mm healing cap was placed at the #5 site and a Uniqa 4.0 X 5 mm healing cap placed at the #3 site.
Healing caps were hand tightened. The rotary instrument was carefully used to remove the tissue over the implants, and then the area was cleansed using consepsis. The healing caps were then placed and hand-tightened.
About three weeks is being allowed for tissue to form around the caps. The next phase will involve taking a final impression for the implant bridge.
9/03/24
Final impressions
Final impressions 3 weeks post 2nd stage surgery. The patient came in today for a final impression of the implant bridge at sites #3 to #5. The healing caps were removed. Open tray impression transfer abutments were placed on the #3 implant while a closed tray transfer abutment was used for #5. An open and closed tray impression was then taken with PVS using a full arch tray.
Why combine open and closed tray impression copings? Using multiple closed tray impression copings can make impression removal difficult, potentially leading to a “locked” impression. However, combining open and closed tray copings minimizes this risk. I chose a closed tray coping for #5 due to difficult access. The combination of these transfer coping types simplifies the impression procedure.
The healing caps were cleaned with 0.12% chlorohexidine gluconate and placed back onto the implants with 10Ncm torque. Shade A3 was recorded for the new restoration. An alginate impression of the opposing arch was taken, as well as a blue bite registration. Postoperative instructions were provided once again.
9/30/24
Delivering the implant bridge at sites #3-5 (UNIQA)
Patient came for delivery of implant supported bridge at sites #3-6.
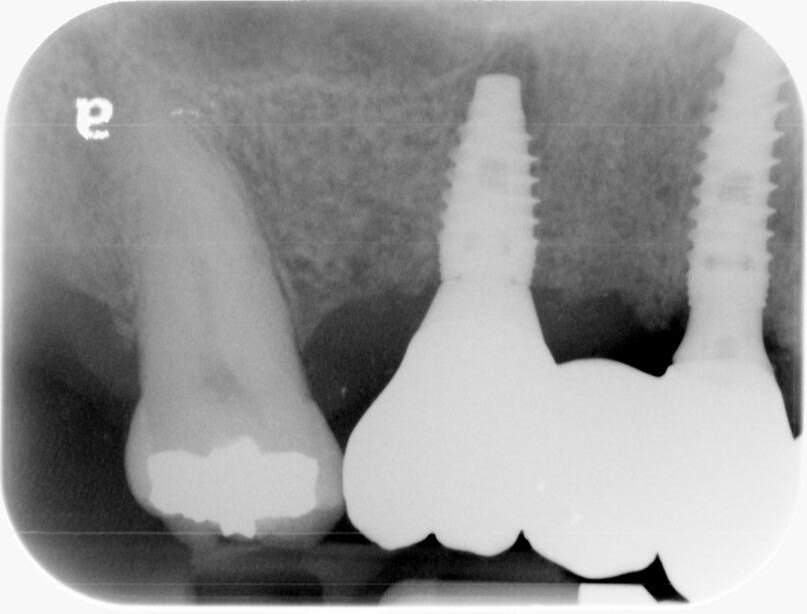
The healing caps were removed and the titanium custom abutments were carefully seated with a positioning stent and torqued to 30Ncm. The zirconia bridge was then tried in, paying close attention to the occlusion, proximal contacts, marginal fit, and any indication of gingival tissue blanching. After a minor occlusal adjustment, cotton pellets were placed into the titanium abutment access holes. Triple A ointment was applied to the external aspect of the
crown margins. Finally, the bridge was seated using Panavia SA dual cure cement, and any residual cement was carefully removed. The patient was absolutely thrilled with
the esthetic outcome. A final PA was captured, and floss instruction was provided. It was a joy to see how pleased the patient was with their aesthetic results.
Summary Table of the Clinical Case
| Parameter | Result |
|---|---|
| Implant status | Osseointegration is complete |
| Healing phase | Full recovery |
| Bone tissue status | No bone loss |
| Stability | Good implant stability |
| Aesthetics | Good |
| Restoration status | Zirconium cement retained bridge with custom titanium abutment |