Pleasant “side effects” of immunotherapy are improved gum health: promising studies in mice
p>Periodontal disease (PD) is a widespread serious inflammatory disease that affects the gums and bones that support the teeth. It has long been known that bacterial infections cause inflammation leading to bone destruction in PD, but new research has revealed a deeper, immune cause. By targeting the immune system, not just the bacteria, we can not only treat periodontal disease, but also potentially prevent the progression of Parkinson’s disease because the inflammatory mechanisms in this disease are similar. This article reviews groundbreaking discoveries in the field of immune modulation as an innovative approach to combat this common but severe disease.
Understanding the Immune Response in Periodontal Disease
PD is traditionally treated by targeting bacterial buildup through dental cleanings, antimicrobial treatments, and rigorous oral hygiene. However, these methods address only part of the issue. PD occurs when the body’s immune response, specifically inflammation, becomes overactive due to the presence of bacteria in the gum tissue. The immune system’s role in driving bone loss has become an essential focus in recent research, which shows that bacteria alone do not cause the disease; rather, they trigger an immune response that spirals into chronic inflammation.
In PD, the immune system’s macrophages—cells responsible for fighting infections and removing dead cells—play a central role. Typically, macrophages come in two types: M1, which promotes inflammation, and M2, which promotes tissue repair and reduces inflammation. In patients with PD, M1 macrophages dominate, leading to prolonged inflammation and bone destruction.
Diagram: PD Progression and Macrophage Shifts
Microparticle-Based CCL2 Therapy: A Novel Approach
A recent study by researchers at the University of Pittsburgh has shown that modulating the immune system can also halt or reverse the progression of Parkinson’s disease. At the heart of this new approach is the immunomodulatory compound CCL2, which alters the immune response of the gums. CCL2 switches macrophages from inflammatory type M1 to anti-inflammatory type M2, reducing tissue damage and promoting bone regeneration.
How CCL2 Works
Using microparticles to deliver CCL2 directly to affected gum tissue offers a sustained release of the compound, providing prolonged immune modulation. The microparticles are engineered to localize around the diseased tissue, preventing further bone loss while encouraging bone repair. By addressing the underlying immune dysfunction rather than just bacterial infection, this method offers a revolutionary way to manage PD.
Key Benefits of CCL2 Therapy:
- Reduces Inflammation: CCL2 shifts the immune response from pro-inflammatory to anti-inflammatory.
- Promotes Bone Repair: The therapy not only halts bone loss but actively encourages regeneration.
- Alters the Oral Microbiome: By influencing the immune environment, CCL2 reduces bacterial load and prevents harmful bacterial overgrowth.
Research Insights: Preventing and Treating PD
The study showed that administering CCL2 microparticles in three different scenarios—preventative treatment, during active disease, and as part of recovery—yielded significant results. Whether delivered before bacterial buildup or after bone loss had begun, CCL2 prevented the progression of PD and aided in recovery by promoting bone regrowth.
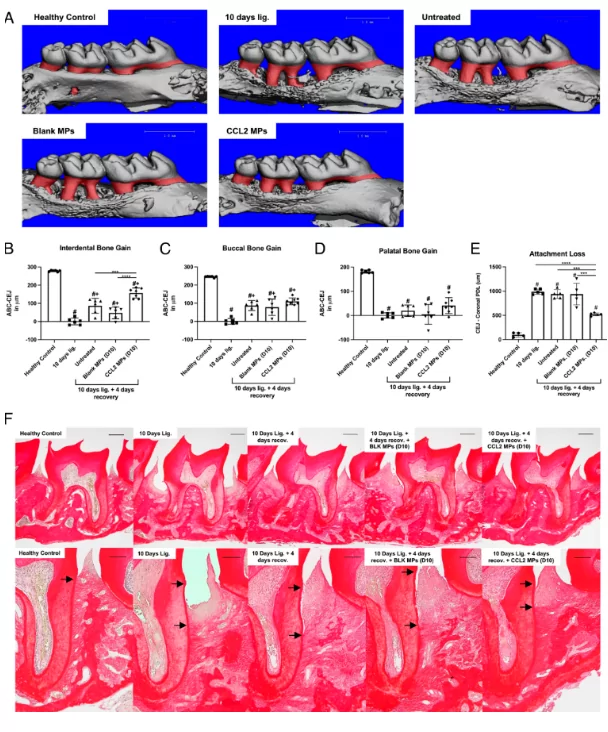
Local sustained delivery of CCL2 MPs accelerates periodontal repair during murine PD resolution. We assessed alveolar bone gain and periodontal ligament organization after 4 d from ligatures removal with or without PLGA MPs local delivery (recovery period) following ligature PD induction for 10 d (Reparative therapeutic approach). (A) Representative 3D images of the reconstructed micro-CT scans from each experimental group in the reparative therapeutic approach experiment. Bone gain was calculated by subtracting the ABC-CEJ measurements in mice that had undergone 4 d recovery from the corresponding average measurements of the 10 d ligature only mice on each aspect. (B–D) Alveolar bone gain quantification on the interdental (B), buccal (C) and palatal (D) aspects of the previously ligated maxillary second molar following the 4 d recovery period. One-way ANOVA with a post hoc Tukey test was performed to determine statistical significance, where *P < 0.05, **P < 0.005, ***P < 0.0005 and ****P < 0.0001, #: significantly different from “Healthy control group” and +: significantly different from the “10 d ligature group.” N = 6 to 7 mice. (E) Quantification of attachment loss, which is the linear distance between the cemento-enamel junction (CEJ) and the most coronal level of the PDL collagen fibers. (F) Representative 4× (Top row) and 10× (Bottom row) images of the picrosirius red staining of tissue sections from the reparative approach experiment with black arrows pointing to the CEJ and the level of the most coronal PDL collagen fibers on the root surface.
This treatment’s impact was seen both in the immune system’s response and the oral microbiome, as CCL2 also reduced bacterial populations associated with PD. This shift illustrates the close connection between immune modulation and microbial control, underscoring the role of immune responses in shaping bacterial environments.
Comparison of PD Treatments
| Treatment Type | Mechanism of Action | Impact on Bone Loss | Impact on Bacteria | Immunomodulation |
| Traditional Dental Cleaning | Removes bacterial buildup | Prevents inflammation reoccurrence temporarily | No direct effect | No |
| Antimicrobial Therapy | Targets bacterial infection | Reduces inflammation linked to infection | Reduces bacteria load | No |
| CCL2 Microparticle Therapy | Modulates immune response | Prevents bone loss and promotes regeneration | Lowers bacterial load | Yes |
The Future of PD Treatment: Combining Approaches
Although CCL2 therapy offers a powerful new tool for combating PD, it is likely to be used in combination with traditional treatments such as antimicrobial therapy and dental cleanings. For the majority of PD patients, periodic dental cleanings are sufficient to manage inflammation. However, for patients with aggressive PD, where bone destruction persists despite cleanings and antimicrobial treatments, CCL2 therapy could provide an effective adjunct.
CCL2 therapy holds promise as a game-changer for those who experience severe, persistent PD that does not respond to standard care. By addressing both immune dysfunction and bacterial presence, this dual approach offers hope for better long-term outcomes.
Broader Implications: Immune Modulation and the Microbiome
The findings from this research extend beyond periodontal disease. The oral cavity is a valuable model for studying the interaction between the immune system and microbiome, as it is more easily accessible than other parts of the body. Insights gained from this research could influence treatment strategies for other diseases driven by chronic inflammation, such as inflammatory bowel disease or rheumatoid arthritis.
Moreover, this research highlights the importance of considering immune system responses when developing treatments for diseases traditionally linked to bacterial infection. The ability to manipulate the immune environment to control bacterial populations opens new avenues for treating a wide range of inflammatory conditions.
Conclusion
The discovery that targeting the immune system with therapies like CCL2 microparticles can prevent or treat periodontal disease marks a paradigm shift in how we approach PD treatment. By addressing the root cause of inflammation and bone loss—immune system overactivation—this innovative approach offers hope for patients with aggressive forms of PD who do not respond to conventional treatments.
The combination of immune modulation and bacterial control heralds a new era in periodontal disease management, with potential implications for other inflammatory diseases as well. As further clinical trials unfold, we can anticipate that CCL2 therapy will become a key component of comprehensive PD treatment strategies, offering more effective and lasting relief for patients worldwide.
Sources
- ScienceDaily – Immunotherapy for gum disease? Study in mice shows promise – September 30, 2024;
- PNAS – Therapeutic delivery of CCL2 modulates immune response and restores host–microbe homeostasis – August 26, 2024








