Nanotechnology Revolutionizes Dental Implant Surface Treatment
The evolution of dental implants aims to reduce the time of osseointegration and decrease the number of rejections. The classic approach is considered to be the mechanical treatment of the titanium surface with a stream of solid particles followed by acid etching to remove the remnants of the abrasive substance. As a result, the surface becomes rough with many microscopic indentations and irregularities. The success rate of implantation ranges from 96 to 99%, depending on the patient’s clinical health and the dentist’s qualifications. The average time for osseointegration is from 6 to 12 weeks, which is significantly less than in the era of smooth (milled) implants. However, even a six-week period can cause considerable discomfort to the patient.
In this article, we will briefly discuss the application of nanotechnologies and improvements in the quality of titanium implant surfaces.
Main quality criteria for the implant surface
- Surface relief and hydrophilicity of the surface. The surface should create favorable conditions for contact with platelets and blood proteins. Mesenchymal stem cells appear rapidly at the point where the implant surface contacts the bone tissue. Then they differentiate into osteoblasts and complete healing and integration of the titanium implant with the bone tissue occur. Hydrophilic properties of the surface contribute to the acceleration of blood clotting and accelerate the adsorption of proteins such as fibronectin, vitronectin.
- Chemical Composition. Not only is purity from harmful impurities important, but the type of surface itself also matters. For example, pure titanium or a nanocoating of calcium phosphate (CaP), which mimics the chemical composition of living bone, is good for accelerating osseointegration. Moreover, not only the properties improving osseointegration are important, but also the antibacterial properties of the surface itself.
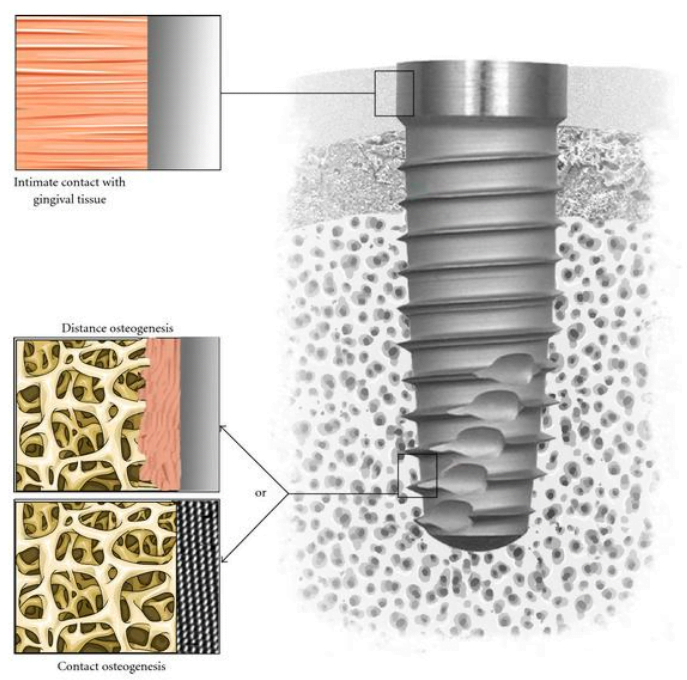
All this affects the quality of the connection between the implant and the bone tissues. Pay attention to the illustration below.
Contact osteogenesis is considered successful when there is no layer of fibrous tissue between the surface of the implant and the bone tissue. The formation of a quality connection at the level of soft tissues is considered no less important. The gingival cuff prevents the penetration of bacteria into internal structures, and therefore the risk of peri-implantitis is minimized.
What problem do nanotechnologies solve in the processing of implant surfaces
The surface of metallic implants should possess biological properties that promote protein adsorption, cell adhesion and differentiation, as well as tissue integration with implant surfaces.
These biological properties are related to the chemical composition, wettability, and roughness of the surface, and these qualities can only be controlled at the protein and cellular level in the nanometer range. But the task of researchers is not only to control and observe biological processes but also to create surfaces with predictable topography and chemical composition that promote the easing of contact osteogenesis.
What Technologies Allow Control of the Topography of Dental Implant Surfaces at the Nanometer Level
New implant surfaces with predictable properties can be obtained using several methods of the electronics industry:
- Electron beam or ion beam lithography;
- Ion implantation – acceleration of the material ions by an electric field;
- Anodization allows growing nanotubes on the titanium surface – by controlling the time and voltage of the electric field, it is possible to get nanotubes in the range of sizes from 20 to 120 micrometers;
- Radiofrequency plasma processing.
With these technologies, surfaces with controllable characteristics at the nanometer scale can be created. These surfaces can then be tested with high-throughput biological assays in vitro. For instance, they can test the quality of specific protein adsorption, cell adhesion, and stem cell differentiation.
A series of checks allows determining the ideal surface for a specific biological reaction. After in vitro screening, nanostructured surfaces can be tested on animal models to confirm the hypothesis in a complex in vivo environment.
Technologies for applying a layer of hydroxyapatite and related calcium phosphates also hold promise. These minerals are part of bone tissue, and a coating of them provides osteoconductive properties to the surface of titanium implants.
After implantation, the CaP coating in the area around the implant begins to dissolve, which increases the ionic strength and blood saturation. This, in turn, led to the deposition of biological nanocrystals of apatite on the surface of the implants. This biological apatite layer includes proteins and promotes adhesion of osteogenesis precursor cells that emerge from the extracellular matrix of bone tissue. Moreover, studies have shown that osteoclasts, the bone-resorbing cells, can enzymatically break down CaP coatings and create resorption pits on the coated surface.
Finally, the presence of CaP coatings on metals promotes earlier osseointegration of implants with direct bone attachment compared to uncoated surfaces. The challenge for technologists is to create CaP coatings that dissolve at the same rate as new bone formation, ensuring direct contact of bone tissue with the implant surface after the complete dissolution of the hydroxyapatite layer.
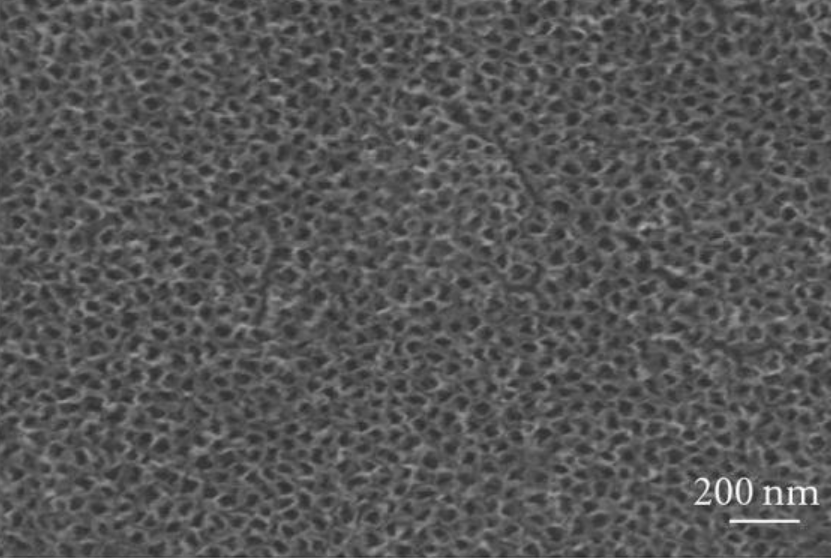
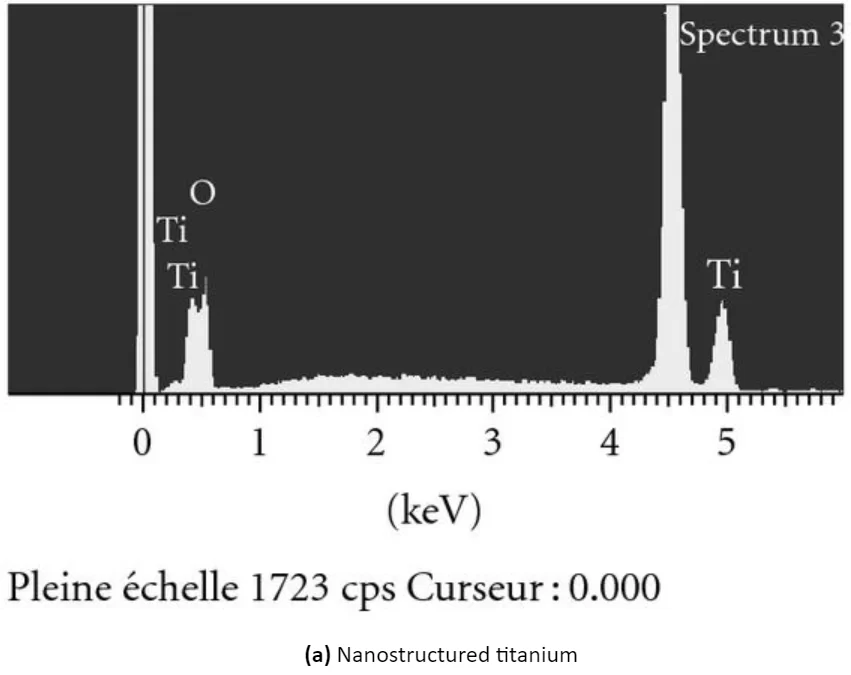
You can see the appearance of the surface of modified titanium without coating and titanium with a CaP coating in the illustration below:
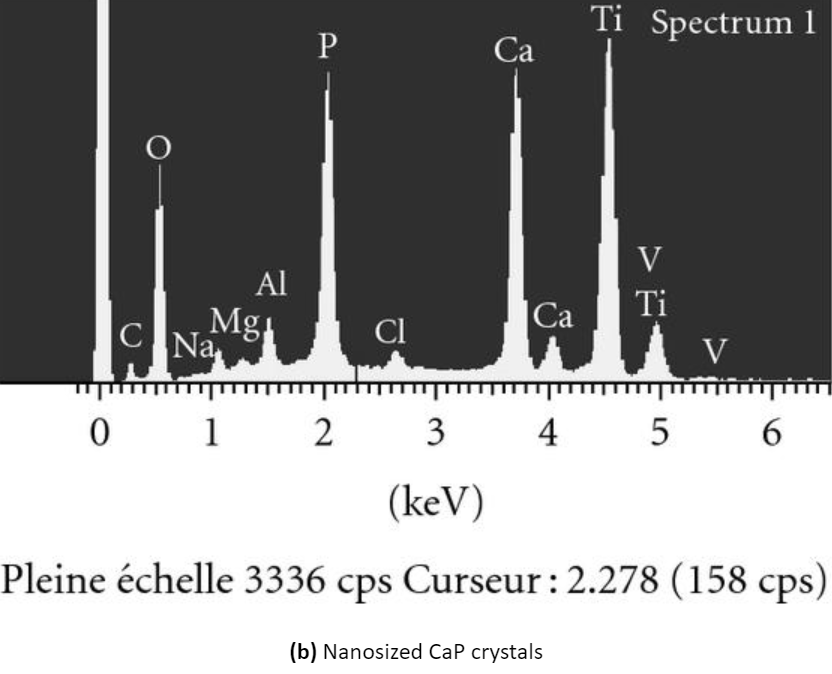
Scanning electron micrographs and energy dispersive analysis for x-ray of (a) nanostructured titanium surface obtained by anodization and (b) nanosized thin calcium phosphate (cap) coating on titanium produced by electrochemical deposition. Note the regular array of tio2 nanopores of approximately 100 nm in diameter and the nanosized cap crystals on titanium surfaces.
Forecasts and prospects for the use of nanotechnologies in the production of dental implants
Numerous studies indicate that surfaces controlled at the nanometer level positively influence early healing processes, such as protein adsorption, thrombus formation, and cell behavior at the interface between living tissue and implant surfaces. This is beneficial for the migration, attachment, and differentiation of MSCs on the implant surface. However, the search for the ideal solution continues with ongoing trials and research. Nevertheless, the existing results are promising and hold practical significance.
Additional information about the use of nanotechnologies in modifying the surface of dental implants: