Innovative Noninvasive Test Revolutionizes Oral Cancer Detection
In the realm of medical diagnostics, the detection of oral cancers and precancerous mouth lesions has long been a challenging endeavor. Traditional methods such as biopsies, while effective, come with significant drawbacks including expense, invasiveness, patient stress, and the potential for complications. Moreover, the need for repeated screenings of the same lesion renders biopsies impractical in many cases.
A Breakthrough Discovery
However, a groundbreaking solution has emerged from the collaborative efforts of researchers, spearheaded by a clinician scientist at the Case Western Reserve University School of Dental Medicine. Their pioneering work has led to the development of a noninvasive, cost-effective test that promises to revolutionize the detection of oral cancer, monitoring of precancerous lesions, and the determination of when a biopsy is necessary.
Understanding the Test
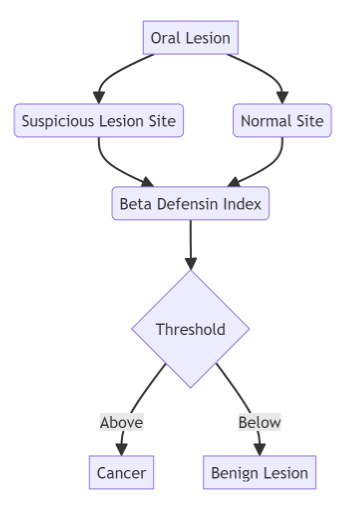
At the heart of this innovation lies a novel scoring system based on the levels of two key proteins present in cells obtained from suspicious oral lesions. The first protein, human beta defensin 3 (hBD-3), exhibits high expression levels in early-stage oral cancer, while the second protein, hBD-2, remains low or unchanged. By analyzing the ratio of hBD-3 to hBD-2 at the lesion site compared to the normal site, a score known as the beta defensin index (BDI) is generated.
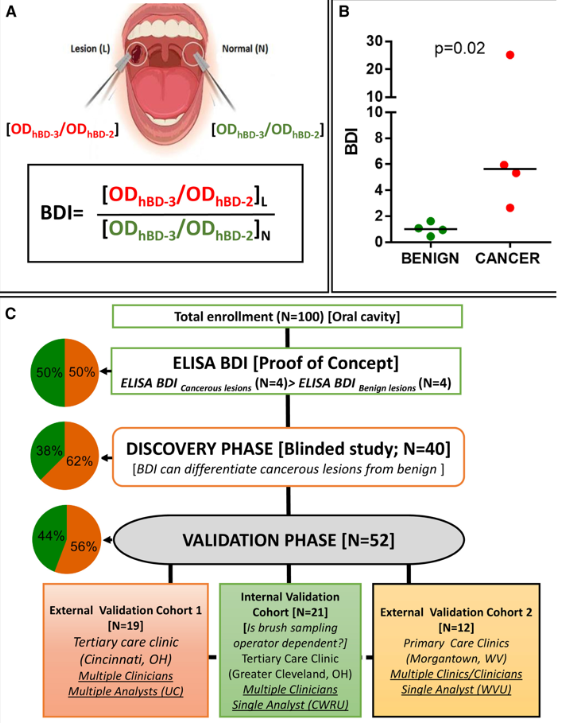
(A) Cartoon presentation and formula for the beta-defensin index (BDI). (B) Proof of concept for the BDI. Cytobrush samples were collected from four OSCC and four non-cancerous lesions. Diagnosis was determined by biopsy followed by pathology review. BDI values, as determined in (A), were calculated following ELISA. (C) Flow diagram of ELISA-based BDI biomarker for proof-of-concept, discovery, and validation studies. Pie chart shows the percentage of non-cancerous/benign lesions (green) and cancerous lesions (orange) for each cohort. UC, University of Cincinnati; CWRU, Case Western Reserve University; WVU, West Virginia University.
Validation and Reliability
The reliability of this scoring system, crucial for widespread adoption, has been independently validated using rigorous protocols at multiple institutions including Case Western Reserve University, the University of Cincinnati Medical Center, and the West Virginia University School of Dentistry. This validation process underscores the robustness and accuracy of the test across diverse patient populations and clinical settings.
Implications for Public Health
The implications of this breakthrough extend far beyond the realm of diagnostics. Head and neck cancer (HNC), of which oral cancer comprises a significant portion, ranks as the seventh most prevalent malignancy globally. Particularly alarming is the rising incidence of HNC in developing countries, where access to quality healthcare services remains limited. By streamlining the diagnostic process and reducing the need for invasive procedures, this test has the potential to make a profound impact on public health outcomes worldwide.
From Lab to Clinic
One of the most promising aspects of this innovation is its practicality in diverse healthcare settings. The lab-based approach, now patented, has the potential to reduce the need for biopsies in primary care clinics by a staggering 95%. This not only alleviates the burden on patients but also optimizes healthcare resources by directing biopsies only to those who truly require them.
Advancing Point-of-Care Technology
Building upon the success of the lab-based approach, researchers are actively developing a point-of-care (POC) device in collaboration with engineering experts. This portable diagnostic tool will enable real-time analysis of the protein ratio, providing results within minutes directly in a clinical setting. The expedited turnaround time offered by the POC device holds immense promise for improving patient outcomes and streamlining healthcare delivery.
Future Prospects
As this groundbreaking technology continues to evolve, it has the potential to redefine the standard of care for oral cancer screening and diagnosis. With ongoing support from government agencies and research institutions, including the National Institute of Dental and Craniofacial Research and the National Cancer Institute, the journey from bench to bedside is well underway. By harnessing the power of innovation and collaboration, we are poised to usher in a new era of precision medicine in the fight against oral cancer.
In conclusion, the development of this noninvasive test marks a significant milestone in the ongoing battle against oral cancer. By offering a reliable, cost-effective, and patient-friendly alternative to traditional biopsy methods, it has the potential to save lives and improve outcomes for countless individuals worldwide. As we continue to harness the power of scientific innovation, we move closer to a future where oral cancer is not just treatable but preventable.
Sources
- ScineceDaily – New method to test for oral cancer – March 4, 2024;
- 50 Cell Reports Medicine – Beta-defensin index: A functional biomarker for oral cancer detection – March 4, 2024