The Connection Between Periodontal Disease and Rheumatoid Arthritis: Insights from Recent Research
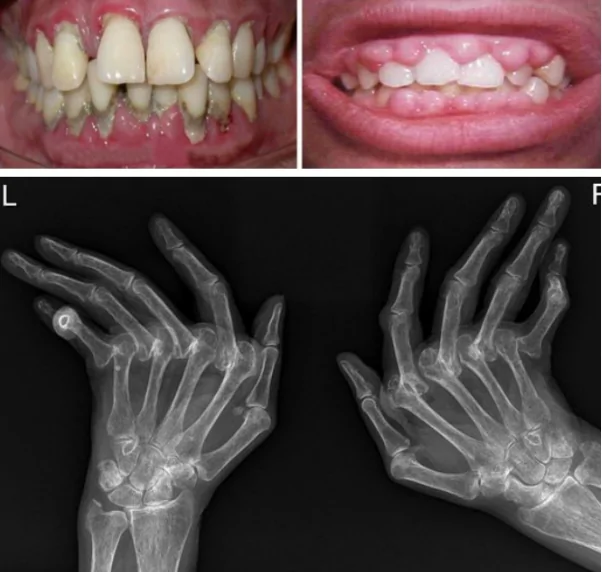
Periodontal disease, a common dental condition affecting millions globally, has long been associated with oral health complications, but emerging research reveals that its impact may extend beyond the mouth. In a recent study published August 15, 2024 in the International Journal of Dental Science, a research team from Tokyo Medical and Dental University (TMDU) in Japan shows that the bacterial pathogen Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans), a leading cause of periodontal disease, may exacerbate the symptoms of rheumatoid arthritis (RA). This discovery is shedding light on the intricate link between oral infections and systemic diseases, with potential implications for novel therapeutic approaches to managing RA and other inflammatory conditions.
The Role of Aggregatibacter actinomycetemcomitans in Periodontal Disease
Periodontal disease, characterized by the inflammation of the gums and surrounding tissues, typically results from the accumulation of bacterial biofilm, or plaque, on the teeth. Among the various bacterial species implicated in this process, A. actinomycetemcomitans has garnered particular attention due to its aggressive nature and its ability to evade the immune system.
This bacterium not only causes localized inflammation but also triggers immune responses that may have far-reaching effects on the body.
Research has shown that A. actinomycetemcomitans can provoke an overactive immune response, leading to increased levels of pro-inflammatory cytokines, including interleukin-1β (IL-1β). These cytokines contribute to the destruction of periodontal tissues and are also believed to play a role in the progression of systemic conditions like rheumatoid arthritis.
Rheumatoid Arthritis and the Inflammatory Cascade
Rheumatoid arthritis is an autoimmune disease that primarily affects the joints, causing inflammation, pain, and swelling. It is a chronic condition that can lead to joint damage and disability if left untreated. The underlying cause of RA involves the immune system mistakenly attacking the synovial tissues that line the joints, leading to the release of inflammatory mediators such as cytokines and enzymes that break down cartilage and bone.
The connection between periodontal disease and RA lies in the shared inflammatory mechanisms between the two conditions. Both diseases involve the activation of immune cells, including macrophages, which release inflammatory cytokines like IL-1β and tumor necrosis factor-alpha (TNF-α). The presence of A. actinomycetemcomitans in periodontal tissues can exacerbate these inflammatory processes, leading to a worsening of RA symptoms.
Key Findings: Macrophages and Caspase-11 in Disease Progression
Recent studies conducted on mouse models of arthritis have provided critical insights into the molecular pathways linking periodontal disease and rheumatoid arthritis. In particular, macrophages—immune cells that play a key role in the body’s response to infection—have been identified as major contributors to the worsening of RA symptoms in the presence of A. actinomycetemcomitans.
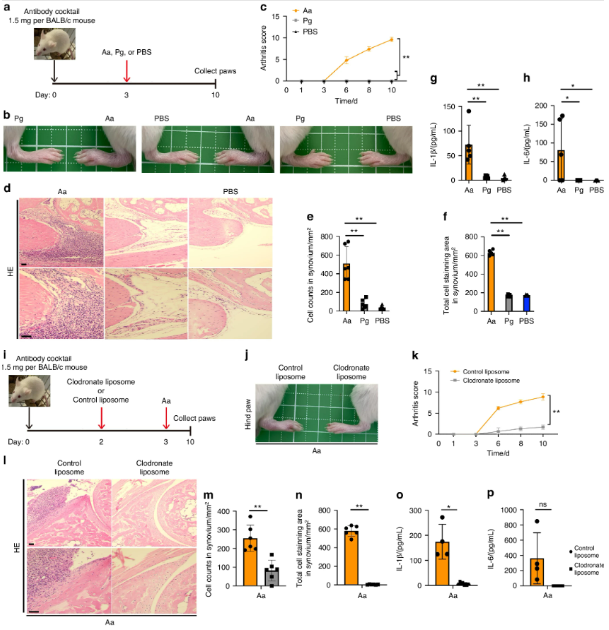
A. actinomycetemcomitans exacerbates arthritis in a CAIA model in a macrophage-dependent manner. a–h BALB/c mice were intraperitoneally injected with 1.5 mg of anti-collagen antibody on day 0 and with A. actinomycetemcomitans ATCC29522 (Aa), P. gingivalis ATCC33277 (Pg) or PBS on day 3. The arthritis scores were then monitored for 10 days. All paws were collected on day 10 for HE staining or cytokine analysis (n = 5 mice per group). a Schematic of CAIA model infected with A. actinomycetemcomitans or P. gingivalis. b Representative photographs of hind paws. c Arthritis score. d Representative HE staining of hind paws. Scale bar, 100 μm. e Cell number in 1 mm2 synovium. f Stained cell area in 1 mm2 synovium. g, h ELISA analysis for IL-1β and IL-6 release in paws i–p BALB/c mice were intraperitoneally injected with 1.5 mg of anti-collagen antibody on day 0, followed by the injection of liposomes containing clodronate or PBS on day 2 and A. actinomycetemcomitans (Aa) on day 3. The arthritis scores were monitored for 10 days. The paws of all the mice were collected on day 10 for HE staining or cytokine analysis (clodronate liposome, n = 8; control liposome, n = 4). g Schematic of CAIA model infected with A. actinomycetemcomitans and administered 10 μL/g of liposomes containing clodronate or PBS. Scale bar, 100 μm. h Representative photographs of hind paws. i Arthritis score. j Representative HE staining of hind paws. k, l ELISA analysis showing the release of IL-1β and IL-6 in paws. c, k ∗∗P < 0.01 indicates a statistically significant difference using a one-way ANOVA with the Tukey test (ns, not significant). e–h, m–p ∗P < 0.05 and ∗∗P < 0.01 indicate a statistically significant difference using a t-test (ns, not significant)
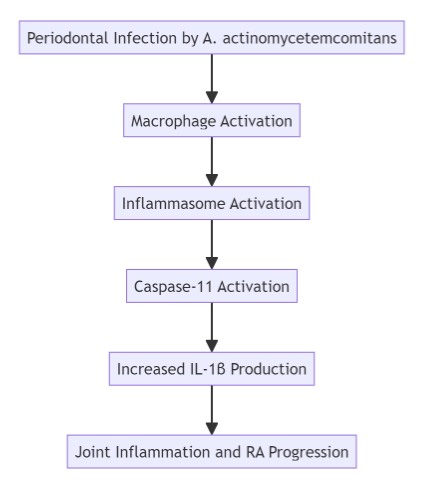
When the bacterium infects periodontal tissues, it triggers the activation of the inflammasome, a multiprotein complex responsible for initiating the inflammatory response. The inflammasome, in turn, activates caspase-11, a protein that promotes the release of IL-1β. This cytokine plays a central role in the inflammatory cascade, driving both the progression of periodontal disease and the exacerbation of RA symptoms.
In experiments using mice deficient in caspase-11, researchers observed a significant reduction in arthritis symptoms, suggesting that targeting caspase-11 could be a promising strategy for managing RA in patients with periodontal disease. Furthermore, the depletion of macrophages in these models led to a marked decrease in joint inflammation, further supporting the idea that these immune cells are key players in the disease process.
Clinical Implications and Future Directions
The findings from these studies offer new avenues for the development of treatments aimed at mitigating the impact of periodontal disease on rheumatoid arthritis. By targeting the inflammasome and caspase-11 pathways, it may be possible to reduce the inflammatory burden in patients with both conditions, leading to improved outcomes and a better quality of life.
Moreover, these insights highlight the importance of oral health in the management of systemic diseases. Regular dental care and the prevention of periodontal infections could play a crucial role in reducing the risk of RA exacerbation, particularly in individuals who are genetically predisposed to autoimmune conditions.
Potential Therapeutic Strategies
- Inflammasome Inhibitors: Given the central role of the inflammasome in driving inflammation in both periodontal disease and RA, targeting this pathway with specific inhibitors could offer a new therapeutic approach. Drugs that block the activation of the inflammasome or its downstream effectors, such as caspase-11, may help to alleviate symptoms in patients with co-occurring periodontal disease and RA.
- Macrophage Modulation: Therapies that deplete or modulate the activity of macrophages could also prove beneficial. Clodronate, a chemical agent that depletes macrophages, has already shown promise in animal models, suggesting that this strategy could be explored further in clinical trials.
- Cytokine Blockers: Blocking the action of pro-inflammatory cytokines like IL-1β and TNF-α is already a cornerstone of RA treatment. However, combining these therapies with interventions that target periodontal pathogens may enhance their efficacy and reduce the risk of RA flares triggered by oral infections.
Conclusion: A Path Forward for Integrated Care
The growing body of evidence linking periodontal disease with rheumatoid arthritis underscores the need for an integrated approach to healthcare that considers both oral and systemic health. By addressing the underlying bacterial infections that contribute to chronic inflammation, we may be able to improve outcomes for patients with RA and reduce the burden of this debilitating disease.
As research continues to unravel the complex interactions between periodontal pathogens and autoimmune conditions, new opportunities for treatment are likely to emerge. In the meantime, maintaining good oral hygiene and seeking timely dental care remain critical steps in preventing the escalation of inflammatory diseases.
Together, these efforts will not only advance our understanding of the connections between periodontal disease and RA but also pave the way for more effective, targeted treatments that address the root causes of inflammation.
Sources
- ScienceDaily – Shedding light on how oral bacteria can aggravate rheumatoid arthritis – September 5, 2024
- IJOS – Caspase-11 mediated inflammasome activation in macrophages by systemic infection of A. actinomycetemcomitans exacerbates arthritis – August 15, 2024