Biomimetic Materials for Dental Implants: How Mimicking Natural Biological Properties Enhances Adhesion and Accelerates Integration with Surrounding Tissues
Biomimetics is the attempt to replicate the properties of living tissues using technology in order to seamlessly connect living tissues with non-biological objects. This article focuses on the application of biomimetic materials in the field of dentistry.
To enhance cellular reactions with biomimetic objects, several important properties come into play, including:
- Chemical composition: It should closely resemble or be highly compatible for contact with living tissues.
- Wetting ability: Critical for adhesion with protein structures.
- Roughness: Important for integration with bone tissue cells.
- Topography: Surface structure, including roughness level and chemical activity.
It is worth noting that the phenomenon of osseointegration, initially described by Professor Branemark, is the result of a series of cellular and molecular events between living cells and the titanium surface. The interaction processes begin immediately after implantation when biological fluids and cells come into contact with the titanium surface. The success of osseointegration is influenced by numerous factors, but we are interested in how the biomimetic properties of surfaces affect the speed and success of osseointegration.
Features and Success Criteria of Osseointegration
Scientists identify three stages of successful osseointegration:
- Initial tissue response within the period of 0 to 4 hours after implant placement. During this period, calcium ions and plasma proteins attach to the implant surface. A blood clot forms, and granulation tissue begins to develop.
- Peri-implant bone formation. Around 3-4 days later, the structure and composition of the blood clot start to change. From day 7 to day 14, mesenchymal stem cells (MSCs) transform into osteoblasts and produce a fibrillar non-collagenous extracellular matrix rich in calcium, phosphorus, osteopontin, and bone sialoproteins, which form the primary bone tissue.
- Peri-implant bone remodeling – the final stage of healing. It begins approximately two weeks after implant placement and continues until full integration. This stage is characterized by an increase in primary bone apposition in direct contact with the original bone and the implant surface. Osteoclasts direct the remodeling process from immature bone to highly mineralized lamellar bone, binding to the mineralized collagen matrix and creating a zone where bone is directly deposited on the implant surface. After three months, the surrounding bone may consist of both lamellar (mature) and non-lamellar bone. The complete integration process can continue for up to one year.
According to modern standards, an implant is considered fully integrated if there is no movement of the implant relative to the bone, even under load. This is a necessary condition for any implant-supported prosthesis.
Information Gathering Strategies and Material Review for Dental Implants
During the data collection process for this article, the databases PubMed, LILACS, Web of Science, and Cochrane Library were explored. The aim was to find research results on how different surface modifications of implants influence the reactions of bone and soft tissues during the healing process. Articles published in the last 30 years in English, Spanish, and Portuguese were taken into consideration.
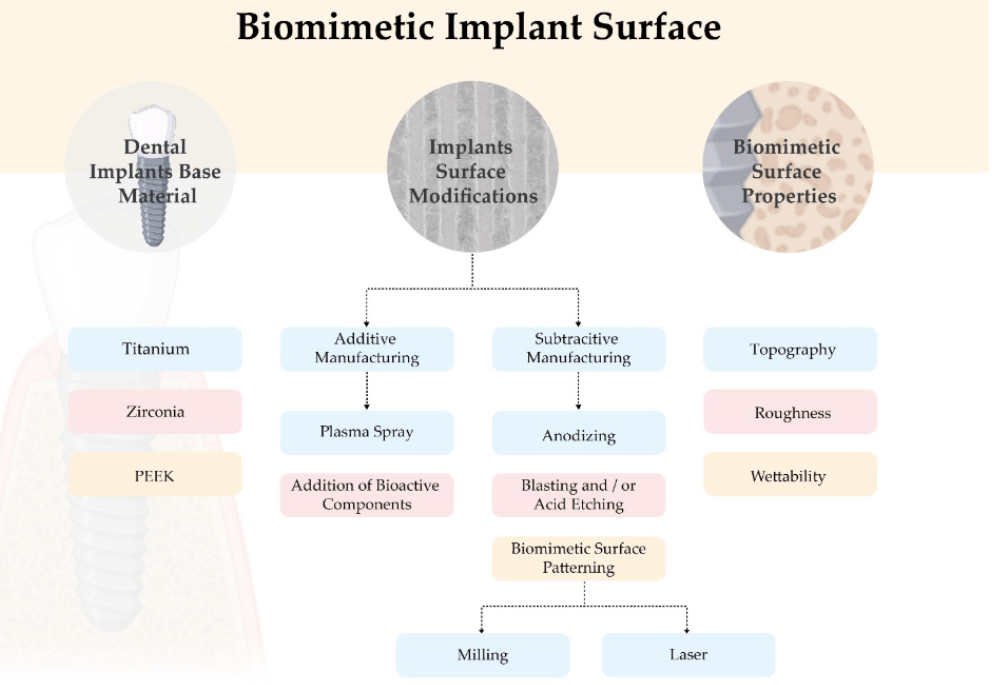
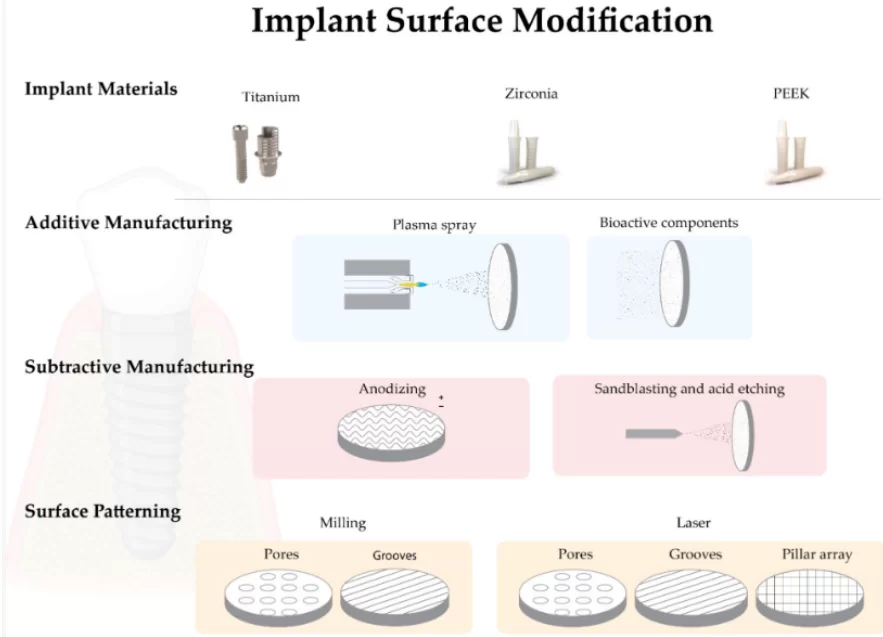
Materials Used for Implant Manufacturing
Broadly, the materials used for dental implants can be categorized into three major groups:
- Metals
- Ceramics
- Polymers
Based on their biological reaction, materials can be classified as:
- Biocompatible: Materials that are well-tolerated by the body.
- Bioinert: Materials that do not elicit a significant biological response.
- Bioactive: Materials that interact with the biological environment, promoting specific reactions.
Different levels of biocompatibility indicate that none of the materials are fully biologically acceptable. Numerous studies have reported cases of peri-implantitis alongside successful integration. Therefore, the goal for scientists is to find the type of material or combinations of materials and surface modifications that minimize the risk of adverse reactions from the body while ensuring maximum functionality for implant-supported prostheses.
An ideal implant surface should exhibit excellent osteoconductivity and biocompatibility, enhancing wound healing around the implant, promoting osteogenesis, and leading to faster and more predictable osseointegration.
Titanium:
Titanium is a metal that forms a thin layer of titanium oxide on its surface when exposed to air. This property is unique to titanium, zirconium, and silicon. The layer of titanium oxide becomes more extensive upon contact with biological tissues, providing excellent biocompatibility for titanium implants. In dentistry, both pure titanium and alloys with small amounts of aluminum, vanadium, niobium, iron, magnesium, or zirconium are used.
According to ASTM classification, there are four grades of titanium based on the content of oxygen, nitrogen, hydrogen, and carbon introduced during the purification process. There are also Grade 5 and Grade 23 alloys containing 6% aluminum and 6% vanadium, respectively.
Titanium and its alloys are the most commonly used materials for dental implants due to their high strength, corrosion resistance, and elastic modulus. However, some concerns are associated with these properties:
- such as hypersensitivity reactions in some patients,
- electrical conductivity,
- differences in elastic modulus,
- and the material’s gray color, which can be an aesthetic drawback.
Therefore, alternative materials have been developed to provide better biological stability while maintaining comparable or improved mechanical properties.
Zirconia:
Zirconia is a crystalline form of zirconium oxide that exists in three forms:
- Monoclinic
- Tetragonal
- Cubic
Tetragonal zirconia polycrystal (TZP) is a ceramic material based on zirconium dioxide primarily in the tetragonal phase and usually stabilized with yttrium oxide (3-6% by weight), known as yttria-stabilized tetragonal zirconia polycrystal (YTZP). The opaque and white color of YTZP, combined with its high fracture strength, flexural strength, thermal stability, low thermal conductivity, chemical resistance, biocompatibility, and low affinity for bacterial colonization, makes it a promising material for tooth implantation.
Overall, various studies have shown that zirconia and titanium have similar integration with bone tissue. However, despite technological advancements in ceramic production, zirconia’s mechanical behavior has limitations, particularly in the aging process. Aging is the process associated with the degradation of zirconium dioxide at low temperatures, leading to the formation of cracks in the ceramic and subsequent failure.
A recent systematic and critical review indicated that zirconia implants are a promising alternative to titanium, exhibiting superior behavior in soft tissues, biocompatibility, and aesthetics while maintaining the same osteointegration capability as titanium. Literature suggests that processed zirconia surfaces demonstrate better or comparable clinical outcomes in bone-implant contact compared to equivalent titanium surfaces. However, other systematic reviews present contradictory results in favor of titanium surface behavior. These reviews did not consider surface parameter assessment as a variable influencing material behavior. Therefore, these conclusions need to be confirmed by integrating adequate surface characteristic evaluations.
Polyether Ether Ketone
PEEK is an organic polymer with a wide range of applications in the medical industry due to its excellent biocompatibility, radiolucency, chemical resistance, low density, and properties similar to human bone.
Its versatility, biocompatibility, chemical resistance to biodegradation, and aesthetic properties make it an interesting polymer for dental implants. However, it falls behind in terms of mechanical properties compared to titanium and zirconia-based implants.
Furthermore, this material possesses physical and mechanical properties similar to human bone, making it suitable for use in orthopedics, traumatology, and spinal implants to minimize stress and, consequently, reduce bone resorption.
Literature has shown that PEEK enhances the behavior of bones and soft tissues, such as early cell adhesion, viability, and proliferation, all of which are associated with increased surface wettability. Additionally, compared to smooth surfaces, porous PEEK surfaces, similar to titanium, promote osteoblast proliferation and differentiation. These findings indicate that chemically inert materials like PEEK do not promote fibrous encapsulation. However, PEEK can be modified to improve biocompatibility, bioactivity, and cellular behavior, with surface topography playing a central role in these mechanisms.
Which surface modification methods show the best results
In recent years, dental implant manufacturers have focused on surface modifications to mimic the characteristics of bone extracellular matrix (ECM), which is considered the most effective way to enhance the speed and quality of osteointegration. To meet these needs, companies have developed a wide range of implant surfaces with complex topography, varying degrees of roughness, and chemical compositions. These modifications can create macro-, micro-, and nanostructures of various shapes on the biomaterial surface, including porous, tubular, or multi-form structures. The optimal roughness size for maximum osteointegration in terms of appearance or distribution on the implant surface is still the subject of intensive research.
Classic surface modification methods can be divided into additive and subtractive methods.
Subtractive methods involve removing a portion of the material from the surface through techniques such as anodization, sandblasting, and/or acid etching.
Additive methods involve adding other materials to the implant surface through processes like plasma spraying, hydroxyapatite coating, calcium phosphate coating, or ion deposition.
Let’s explore how some of these methods work.
- Additive methods of biomimetic surface modification:
- Plasma spraying for titanium surfaces. This method increases surface roughness by depositing hydroxyapatite. Hydroxyapatite particles are introduced into a high-temperature plasma torch and directed onto the titanium surface. The particles condense and fuse into a solid coating. Afterward, the surface is typically subjected to sandblasting to achieve a final roughness with Sa > 2 μm. However, there have been reports of complications related to delamination and marginal bone resorption. Therefore, consensus among specialists now favors implants with moderate roughness instead of plasma spraying.
- Addition of bioactive components. Some components prevent the formation of bacterial biofilm on the surface, while others enhance cell adhesion and osteointegration. Silver, fluoride, zinc, and copper particles possess antibacterial properties. Fluoride is applied in the form of nanoparticles, while silver, copper, zinc, and nickel are incorporated into the titanium surface through anodization, resulting in the integration of nanotubes of these metals. Hydroxyapatite or beta-tricalcium phosphate (β-TCP) has been added to accelerate healing and integration processes. Coating surfaces with these materials have shown good results in terms of biocompatibility, osteoblast differentiation, and osteointegration. However, in in vitro studies, bioactive-modified titanium and zirconia surfaces have shown reduced adhesion, viability, and proliferation of fibroblast cells compared to the pure biomaterial. These coatings have demonstrated low tensile strength (<51 MPa) and fracture toughness (0.28 to 1.41 MPa·m1/2). To overcome these limitations, scientists have developed a new coating method inspired by the natural process of biomimetic mineralization. In this process, calcium phosphate crystals deposited on the titanium surface from simulated body fluids (SBF) form a coating at room temperature.
Several researchers have studied the incorporation of growth factors such as transforming growth factor-beta (TGF-ß) and bone morphogenetic proteins (BMP) as osteoinductive materials for many years. However, despite promising results in bone healing processes, the main limitation is their immediate release rather than gradual release.
Note: Sa refers to the arithmetic average of the absolute value of roughness.
- Subtractive methods of biomimetic surface modification.
- Anodization using strong acids: sulfuric acid (H2SO4), nitric acid (HNO3), phosphoric acid (H3PO4), or hydrofluoric acid (HF). This increases surface roughness and stimulates the formation of an oxide layer. Research results show higher bone-implant contact (BIC) for implants treated with this method compared to mechanical surface treatments. This makes it one of the most promising methods discussed in this article.
- Sandblasting and/or acid etching. These procedures can be performed sequentially or separately. The combined method of sandblasting and acid etching (SBAE) is considered the most promising. The method involves treating the implant surface with abrasive particles of aluminum oxide or titanium, followed by acid etching to remove any remaining abrasive particles from the surface. The acid additionally creates roughness on the titanium surface by dissolving trapped abrasive particles, resulting in the formation of new cavities and indentations. Furthermore, the action of the acid alters the protein adhesion potential, increasing it. This technology is commercially known as SLA (sandblasted, large grit, acid-etched). There are studies confirming that surfaces modified in this way exhibit increased osteoblast differentiation in vitro compared to smooth surfaces. However, most of the research has been conducted on titanium implants. There is currently limited objective data regarding the application of similar methods to zirconia dioxide implants.
Biomimetic Surface Modeling
While we previously discussed methods of indirect surface modification to enhance compatibility with living tissues, let’s now explore surface modeling methods that allow for the precise replication of surface patterns with biological topography. This enables the prediction of predictable reactions from both soft and hard tissues.
Surface topography is considered a critical factor in determining the adhesion, proliferation, and differentiation of human cells. There is confirmed evidence that smooth surfaces promote fibroblast adhesion and the growth of soft tissues, while rough surfaces can be considered enhancers of osteoblast adhesion and bone proliferation. Grooved and microtextured surfaces can provide orientation and directional cues through a phenomenon known as “contact guidance” for osteoblast morphogenesis in a preferred direction.
One of the earliest studies comparing and analyzing cell behavior on predetermined topographies is the research conducted by Chehroudi and colleagues in vitro and in vivo, in which they concluded that surfaces with 10 μm grooves exhibited a higher number of epithelial cell adhesion compared to smooth titanium surfaces. Since then, numerous studies have been published, but most of them pertain to titanium surfaces, with only a few references to zirconia dioxide surfaces. Research findings regarding the optimal forms and sizes of these macrostructures are contradictory. Several methods have been used to create standardized implant surface patterns, such as milling and laser technology. Let’s delve into them in more detail.
- Milling is a mechanical process used to shape and smooth the surface of a material. To the naked eye, a milled surface appears smooth, but it is actually covered with grooves left by the cutting tool and small chips that are periodically removed from the milled surface. It is possible to intentionally create roughness, pores, and grooves of a specified shape and size. These fine grooves, located along the circumference, allow osteogenic cells to attach and establish contact between the implant and bone. Milling is a relatively simple and inexpensive method. However, research studies have produced conflicting results regarding the effect of milling on cell behavior. Therefore, this method cannot be considered 100% universal and effective.
- Laser surface treatment of implants involves the use of various types of lasers, both continuous and pulsed. Solid-state Nd:YAG and gas CO2 lasers have been successfully used to imprint standardized textures on zirconia dioxide implant surfaces. A high-density laser beam is employed to heat, melt, and sublime the material on the implant surface. Laser technology enables the creation of textures at the macro, micro, and nano levels with predetermined properties. The laser increases the roughness of the zirconia implant surface without affecting its crystalline tetragonal phase. Additionally, laser technology is the only method that allows for the rapid creation of the desired structure without direct contact with the surface, thus minimizing the risk of contamination.
Titanium implant surfaces are also modified using laser technology, and there are studies showing that implants with laser-etched grooves exhibit reduced alveolar bone loss and inhibit apical migration of epithelium with strong attachment to the gingival tissue. However, there is not enough research available comparing cell colonization processes on zirconia dioxide surfaces with different structures obtained through various methods. Nevertheless, in vitro studies have shown that zirconia dioxide surfaces with laser-etched patterns using Nd:YAG laser demonstrate better cell behavior compared to non-textured surfaces. It has also been found that the ideal size of the pattern should range from 10 μm to 1 mm.
In conclusion
We have discussed how surface properties such as chemical composition, topography, and roughness influence biological reactions by altering the interaction between implants, proteins, and cells. We have explained and examined methods and materials used to enhance the physical and chemical properties of the surface.
Subtractive methods for improving surface functionality, such as laser treatment, incorporation of antibacterial particles, and incorporation of bioactive particles, have shown the most promising results. Despite challenges with controlled and gradual release of growth factors or other biomolecules, the incorporation of these biological agents into the surface has tremendous potential. However, there is currently significant heterogeneity among methods and research results aimed at determining the impact of surface characteristics on biological tissue processes. Therefore, there is currently no clinical data definitively confirming the superiority of one surface type over another.
Future research should focus on standardizing characteristics and identifying critical variables that provide a clear answer as to how a particular surface type aligns with the concept of clinical success. This will enable the development of increasingly sophisticated and biomimetic surfaces with minimal complications.
By advancing our understanding and optimizing surface modifications, we can create surfaces that closely resemble biological tissues, leading to improved clinical outcomes with minimal complications.
Sources
- National Center for Biotechnology Information – Biomimetic Aspects of Restorative Dentistry Biomaterials 2020 Jul 15;
- MDPI Biomimetic Implant Surfaces and Their Role in Biological Integration—A Concise Review 2022 Jun 6;
- Elias, C.N.; Oshida, Y.; Lima, J.H.C.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242. [Google Scholar] [CrossRef][Green Version]
- Comisso, I.; Arias-Herrera, S.; Gupta, S. Zirconium dioxide implants as an alternative to titanium: A systematic review. J. Clin. Exp. Dent. 2021, 13, e511–e519. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pellegrini, G.; Francetti, L.; Barbaro, B.; del Fabbro, M. Novel surfaces and osseointegration in implant dentistry. J. Inves-Tigative Clin. Dent. 2018, 9, e12349. [Google Scholar] [CrossRef]